How Many Electrons Does P Have
listenit
Mar 20, 2025 · 5 min read
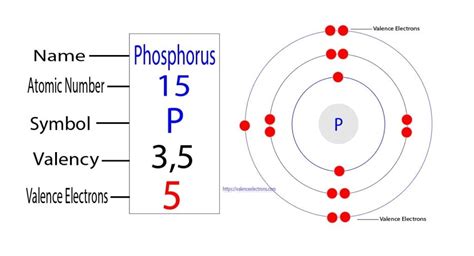
Table of Contents
How Many Electrons Does Phosphorus (P) Have? A Deep Dive into Atomic Structure
Phosphorus (P), a crucial element for life, presents a fascinating case study in atomic structure. Understanding its electron configuration is key to comprehending its chemical behavior and reactivity. So, how many electrons does phosphorus have? The answer is deceptively simple, yet leads to a rich exploration of atomic theory.
The Atomic Number: The Key to Electron Count
The number of electrons an atom possesses is directly determined by its atomic number. This fundamental property, found on the periodic table, represents the number of protons in the atom's nucleus. Since atoms are electrically neutral (unless they're ions), the number of protons equals the number of electrons.
Phosphorus has an atomic number of 15. Therefore, a neutral phosphorus atom possesses 15 electrons.
Electron Shells and Subshells: Organizing the Electrons
While knowing the total number of electrons is crucial, understanding their arrangement within the atom provides a deeper insight into phosphorus's properties. Electrons occupy distinct energy levels, often visualized as shells surrounding the nucleus. These shells are further divided into subshells, each capable of holding a specific number of electrons.
The electron configuration of phosphorus is a concise way to represent this arrangement. It follows the Aufbau principle, which dictates that electrons fill the lowest energy levels first. For phosphorus, the electron configuration is:
1s²2s²2p⁶3s²3p³
Let's break this down:
-
1s²: The first shell (n=1) contains one subshell, the 's' subshell, which can hold a maximum of two electrons. Phosphorus has two electrons in this lowest energy level.
-
2s²: The second shell (n=2) also has an 's' subshell, holding another two electrons.
-
2p⁶: The second shell also contains three 'p' subshells (px, py, pz), each capable of holding two electrons, for a total of six electrons in the 2p subshell.
-
3s²: The third shell (n=3) begins with an 's' subshell holding two more electrons.
-
3p³: Finally, the third shell's three 'p' subshells hold the remaining three electrons. This is where phosphorus's chemical reactivity stems from—its partially filled 3p subshell.
Valence Electrons: The Key to Chemical Bonding
The valence electrons are the outermost electrons, residing in the highest energy level. These electrons are responsible for the atom's chemical behavior and its ability to form bonds with other atoms. In phosphorus's case, the valence electrons are the five electrons in the third shell (3s²3p³).
This partially filled valence shell makes phosphorus highly reactive. It readily gains three electrons to achieve a stable octet (eight electrons in its outermost shell), forming the phosphide anion (P³⁻). Alternatively, it can share electrons through covalent bonds to achieve octet stability.
Phosphorus's Allotropes and Electron Configuration
Phosphorus exists in several allotropic forms, each exhibiting different physical and chemical properties. These variations, however, don't change the fundamental electron configuration of a phosphorus atom. The differences arise from how the phosphorus atoms bond to each other in these different forms:
-
White phosphorus: This highly reactive form consists of discrete P₄ molecules, where each phosphorus atom forms three covalent bonds with its neighbors. The valence electrons are involved in these bonds.
-
Red phosphorus: This less reactive allotrope consists of a polymeric structure with a more complex arrangement of phosphorus atoms, again involving the sharing of valence electrons.
-
Black phosphorus: This is the most thermodynamically stable form and has a layered structure, analogous to graphite. The bonding and electron arrangement are more complex than in white or red phosphorus.
Despite these structural differences, the core electron configuration—1s²2s²2p⁶3s²3p³—remains constant for each phosphorus atom regardless of the allotropic form. The variations in properties are a result of the manner in which these valence electrons are involved in bonding within the different allotropic structures.
Ionization Energies and Electron Removal
The energy required to remove an electron from a phosphorus atom is known as its ionization energy. Phosphorus has multiple ionization energies, corresponding to the removal of successive electrons. The first ionization energy is relatively low compared to elements in later groups, reflecting the relative ease of removing one electron from the 3p subshell. Subsequent ionization energies increase significantly due to the increasing nuclear charge experienced by the remaining electrons.
Understanding these ionization energies is crucial in predicting the chemical behavior of phosphorus under various conditions.
Phosphorus's Role in Biology and its Electron Configuration
Phosphorus is an essential element for life. Its importance stems directly from its electron configuration and the ability of phosphorus to form stable phosphate bonds. These bonds are crucial in:
-
DNA and RNA: The phosphate backbone of these crucial genetic molecules relies heavily on the bonding capabilities of phosphorus.
-
ATP (Adenosine Triphosphate): The energy currency of cells, ATP utilizes high-energy phosphate bonds to transfer energy throughout biological systems. The electron configuration of phosphorus is integral to the stability and reactivity of these phosphate bonds.
-
Bones and teeth: Calcium phosphate is a major component of bones and teeth, providing structural support. Again, the ability of phosphorus to form strong ionic bonds with calcium is crucial.
-
Phospholipids: These are essential components of cell membranes, forming a lipid bilayer. The phosphate groups in phospholipids are responsible for the hydrophilic (water-loving) nature of the membrane's surface.
The chemical reactivity of phosphorus, stemming directly from its electron configuration, is the foundation of its vital roles in biological systems.
Phosphorus in Industry and Technology
Beyond its biological significance, phosphorus finds widespread application in various industries and technologies. These applications often exploit the properties dictated by its electron configuration:
-
Fertilizers: Phosphorus is a key component of fertilizers, essential for plant growth. The reactivity of phosphorus and its ability to form stable phosphate bonds are vital for plant nutrient uptake.
-
Detergents: Phosphates were historically used as water softeners in detergents, but their use is now restricted due to environmental concerns.
-
Matches: Red phosphorus is often used in match heads due to its relatively lower reactivity compared to white phosphorus.
Conclusion: The Significance of 15 Electrons
The seemingly simple answer—phosphorus has 15 electrons—opens a gateway to a wealth of knowledge regarding atomic structure, chemical bonding, and the diverse roles of this element in both biological and industrial contexts. Its 15 electrons, specifically the five valence electrons, are the key to its reactivity and the foundation of its multifaceted importance. Understanding electron configurations is vital for anyone delving into chemistry and its applications.
Latest Posts
Latest Posts
-
What Is 1 3 Times 2 In Cups
Mar 20, 2025
-
Is 87 A Prime Number Or Composite
Mar 20, 2025
-
Seven Times The Sum Of A Number N And Four
Mar 20, 2025
-
What Is 20 Percent Of 400
Mar 20, 2025
-
What Percent Is 4 Out Of 20
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does P Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
