Molar Mass Of Al No3 3
listenit
Mar 15, 2025 · 5 min read
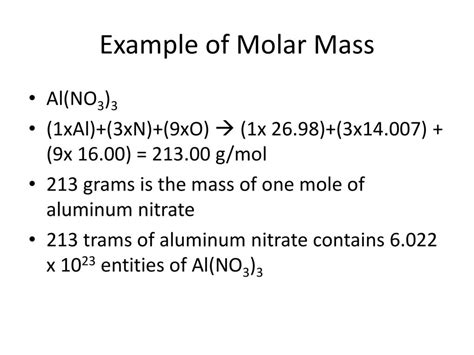
Table of Contents
Calculating the Molar Mass of Al(NO₃)₃: A Comprehensive Guide
Understanding molar mass is fundamental in chemistry, especially when dealing with stoichiometry and chemical reactions. This article delves deep into calculating the molar mass of aluminum nitrate, Al(NO₃)₃, explaining the process step-by-step and highlighting important concepts along the way. We'll explore the underlying principles, address potential confusion, and provide practical examples to solidify your understanding.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles, whether they are atoms, molecules, ions, or formula units. The molar mass is numerically equivalent to the atomic weight (for elements) or molecular weight (for compounds) expressed in grams per mole (g/mol).
Understanding the Chemical Formula of Aluminum Nitrate, Al(NO₃)₃
Before calculating the molar mass, it's crucial to understand the chemical formula: Al(NO₃)₃. This formula tells us that one formula unit of aluminum nitrate contains:
- One aluminum (Al) atom: Aluminum is a metal with an atomic number of 13.
- Three nitrate (NO₃) ions: Each nitrate ion is a polyatomic ion composed of one nitrogen (N) atom and three oxygen (O) atoms.
Step-by-Step Calculation of the Molar Mass of Al(NO₃)₃
To calculate the molar mass of Al(NO₃)₃, we need the atomic masses of aluminum, nitrogen, and oxygen. These values are readily available in the periodic table. For our calculations, we'll use the following approximate atomic masses:
- Aluminum (Al): 26.98 g/mol
- Nitrogen (N): 14.01 g/mol
- Oxygen (O): 16.00 g/mol
Step 1: Calculate the molar mass of the nitrate ion (NO₃⁻)
Each nitrate ion (NO₃⁻) contains one nitrogen atom and three oxygen atoms. Therefore, the molar mass of NO₃⁻ is:
14.01 g/mol (N) + 3 * 16.00 g/mol (O) = 62.01 g/mol
Step 2: Calculate the molar mass of Al(NO₃)₃
One formula unit of Al(NO₃)₃ contains one aluminum atom and three nitrate ions. Therefore, the molar mass of Al(NO₃)₃ is:
26.98 g/mol (Al) + 3 * 62.01 g/mol (NO₃) = 212.98 g/mol + 186.03 g/mol = 213.01 g/mol
Therefore, the molar mass of aluminum nitrate, Al(NO₃)₃, is approximately 213.01 g/mol.
Significance of Accurate Molar Mass Calculation
Precise molar mass calculations are essential for various applications in chemistry, including:
- Stoichiometric calculations: Determining the amounts of reactants and products in chemical reactions requires accurate molar mass values.
- Solution preparation: Preparing solutions with specific concentrations demands precise molar mass calculations to determine the required mass of solute.
- Titration: Molar mass is crucial for calculating the concentration of a solution using titration methods.
- Gravimetric analysis: Determining the amount of an analyte in a sample often relies on molar mass calculations.
Common Mistakes to Avoid
Several common mistakes can lead to inaccurate molar mass calculations. Let's address some of them:
- Incorrectly identifying the chemical formula: Always double-check the chemical formula before beginning calculations. A slight error in the formula can significantly affect the final result.
- Using incorrect atomic masses: Refer to a reliable periodic table for the most accurate atomic mass values. Rounding off too early can lead to significant discrepancies.
- Calculation errors: Carefully check your calculations at each step to minimize errors. Using a calculator with a memory function can help.
- Forgetting the number of atoms/ions: Pay close attention to the subscripts in the chemical formula to account for the correct number of each atom or ion present. This is especially important for polyatomic ions like the nitrate ion in Al(NO₃)₃.
Practical Applications and Examples
Let's look at a few examples to illustrate the practical applications of molar mass calculations involving Al(NO₃)₃:
Example 1: Calculating the mass of Al(NO₃)₃ in a solution.
Suppose you need to prepare 250 mL of a 0.1 M solution of Al(NO₃)₃. How many grams of Al(NO₃)₃ do you need?
- Moles of Al(NO₃)₃: 0.1 mol/L * 0.25 L = 0.025 mol
- Mass of Al(NO₃)₃: 0.025 mol * 213.01 g/mol = 5.33 g (approximately)
Therefore, you would need approximately 5.33 grams of Al(NO₃)₃ to prepare 250 mL of a 0.1 M solution.
Example 2: Determining the amount of a reactant in a reaction.
Consider a reaction where Al(NO₃)₃ reacts with another substance. Knowing the molar mass of Al(NO₃)₃ allows you to determine the number of moles of Al(NO₃)₃ involved in the reaction based on the mass used, facilitating the calculation of the amounts of other reactants or products.
Advanced Concepts and Further Exploration
For a more in-depth understanding, consider exploring these related concepts:
- Percent composition: Determining the percentage of each element in a compound.
- Empirical and molecular formulas: Relating the molar mass to the empirical and molecular formulas of a substance.
- Limiting reactants: Identifying the reactant that limits the amount of product formed in a chemical reaction.
Understanding molar mass is a cornerstone of many chemical calculations. Mastering this concept allows you to confidently tackle a vast array of chemical problems and explore the fascinating world of chemical reactions and stoichiometry with precision and accuracy. By carefully following the steps outlined in this article and practicing with various examples, you can build a strong foundation in this essential chemical concept and enhance your problem-solving skills in chemistry. Remember to always double-check your work and utilize reliable sources for atomic mass data. The accuracy of your calculations directly impacts the reliability of your experimental results and theoretical predictions.
Latest Posts
Latest Posts
-
How To Convert Wavelength To Meters
Mar 17, 2025
-
How Many Valence Electrons In Argon
Mar 17, 2025
-
1 1 X 2 Power Series
Mar 17, 2025
-
What Percent Of 75 Is 40
Mar 17, 2025
-
Translating Graph Up By 4 Units
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Al No3 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
