Lewis Acid And Base Vs Bronsted
listenit
Mar 17, 2025 · 6 min read
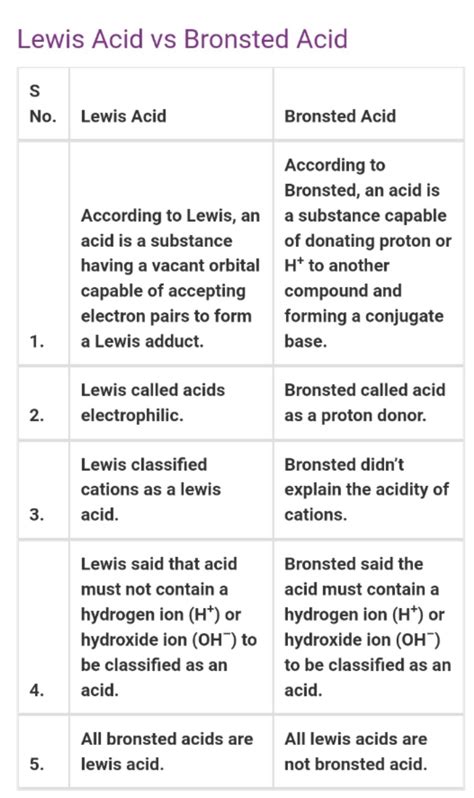
Table of Contents
Lewis Acid and Base vs. Brønsted-Lowry: A Comprehensive Comparison
Understanding acid-base chemistry is fundamental to many areas of chemistry, from organic synthesis to biochemistry. While the Brønsted-Lowry definition of acids and bases is widely taught, the Lewis definition provides a broader and more encompassing perspective. This article delves into a comprehensive comparison of these two prominent acid-base theories, highlighting their similarities, differences, and respective applications.
The Brønsted-Lowry Definition: A Proton's Perspective
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines acids and bases based on proton (H⁺) transfer.
Brønsted-Lowry Acid: The Proton Donor
A Brønsted-Lowry acid is any species that can donate a proton (H⁺) to another species. This donation process results in the formation of the acid's conjugate base. Strong Brønsted-Lowry acids, like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), readily donate their protons. Weak Brønsted-Lowry acids, such as acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), donate protons less readily.
Brønsted-Lowry Base: The Proton Acceptor
A Brønsted-Lowry base is any species that can accept a proton (H⁺) from another species. This acceptance forms the base's conjugate acid. Strong Brønsted-Lowry bases, like hydroxide ion (OH⁻) and alkoxide ions (RO⁻), readily accept protons. Weak Brønsted-Lowry bases, such as ammonia (NH₃) and water (H₂O), accept protons less readily.
Conjugate Acid-Base Pairs
Crucially, the Brønsted-Lowry theory introduces the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs differ by only a single proton. For example, in the reaction between HCl and H₂O:
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
HCl is the acid, Cl⁻ is its conjugate base, H₂O is the base, and H₃O⁺ (hydronium ion) is its conjugate acid.
Limitations of the Brønsted-Lowry Theory
While the Brønsted-Lowry theory is highly useful, it has limitations. It focuses solely on proton transfer and cannot explain acid-base reactions that do not involve protons. For instance, reactions involving Lewis acids and bases, which we will explore next, are not adequately explained by this theory.
The Lewis Definition: A Broader Perspective
Gilbert N. Lewis proposed a more general definition of acids and bases in 1923, expanding the scope beyond proton transfer. The Lewis theory focuses on the donation and acceptance of electron pairs.
Lewis Acid: The Electron Pair Acceptor
A Lewis acid is a species that can accept an electron pair. This acceptance forms a coordinate covalent bond, where both electrons in the bond come from the same atom. Many metal cations (e.g., Al³⁺, Fe³⁺), molecules with incomplete octets (e.g., BF₃), and molecules with polar bonds (e.g., AlCl₃) act as Lewis acids. Their ability to accept electron pairs is often dictated by their electronic structure and electrophilicity.
Lewis Base: The Electron Pair Donor
A Lewis base is a species that can donate an electron pair. This donation also forms a coordinate covalent bond. Many anions (e.g., Cl⁻, OH⁻), molecules with lone pairs of electrons (e.g., NH₃, H₂O), and some neutral molecules with multiple bonds (e.g., alkenes) act as Lewis bases. Their ability to donate electron pairs is linked to the availability of lone pairs and their nucleophilicity.
Examples of Lewis Acid-Base Reactions
The reaction between boron trifluoride (BF₃) and ammonia (NH₃) is a classic example:
BF₃ + NH₃ → F₃B-NH₃
In this reaction, BF₃ acts as a Lewis acid, accepting the lone pair of electrons from the nitrogen atom in NH₃. Ammonia, in turn, acts as a Lewis base, donating its lone pair. The product is a coordinate covalent bond between boron and nitrogen.
Another example involves the formation of a complex ion:
Ag⁺ + 2NH₃ → [Ag(NH₃)₂]⁺
Here, the silver cation (Ag⁺) is the Lewis acid, accepting electron pairs from two ammonia molecules (Lewis bases). The result is a complex ion.
Comparing Brønsted-Lowry and Lewis Theories: Key Differences and Similarities
The most significant difference lies in the focus:
- Brønsted-Lowry: Focuses on proton (H⁺) transfer.
- Lewis: Focuses on electron pair donation and acceptance.
While seemingly distinct, there's a crucial overlap: All Brønsted-Lowry acids are also Lewis acids. This is because a proton (H⁺) can accept an electron pair to form a bond. However, the converse isn't true; not all Lewis acids are Brønsted-Lowry acids. Many Lewis acids, such as BF₃ and AlCl₃, do not contain protons and therefore cannot donate them.
| Feature | Brønsted-Lowry | Lewis |
|---|---|---|
| Definition | Proton transfer | Electron pair donation and acceptance |
| Acid | Proton donor | Electron pair acceptor |
| Base | Proton acceptor | Electron pair donor |
| Scope | Limited to reactions involving protons | Broader scope, includes reactions without protons |
| Examples | HCl, H₂SO₄, NH₃, OH⁻ | BF₃, AlCl₃, Ag⁺, NH₃ |
Applications of Both Theories
Both theories are essential tools for understanding chemical reactions. The Brønsted-Lowry theory is widely used in:
- Acid-base titrations: Determining the concentration of an acid or base using neutralization reactions.
- Buffer solutions: Preparing solutions that resist changes in pH.
- Understanding pH and pKa: Predicting the acidity or basicity of solutions and the relative strengths of acids and bases.
- Biochemistry: Understanding the role of acids and bases in biological systems, such as enzyme catalysis and protein structure.
The Lewis theory, with its broader scope, finds application in:
- Organic chemistry: Understanding reactions involving carbocations, carbanions, and other reactive intermediates.
- Inorganic chemistry: Explaining the formation of coordination complexes and metal-ligand interactions.
- Catalysis: Designing catalysts that involve Lewis acids and bases.
- Materials science: Developing new materials with specific properties based on Lewis acid-base interactions.
Expanding the Understanding: Hard and Soft Acid-Base Theory (HSAB)
Building upon the Lewis theory, the Hard and Soft Acid-Base (HSAB) theory, developed by Ralph Pearson, provides further insight into the reactivity of Lewis acids and bases. This theory categorizes acids and bases as "hard" or "soft" based on their size, charge, and polarizability.
Hard acids are small, highly charged cations with low polarizability, while soft acids are large, less charged cations with high polarizability. Similarly, hard bases are small, less polarizable anions, and soft bases are large, highly polarizable anions or neutral molecules.
The HSAB principle states that hard acids prefer to react with hard bases, and soft acids prefer to react with soft bases. This principle helps predict the stability and reactivity of various compounds and complexes.
Conclusion: A Unified Perspective
While the Brønsted-Lowry and Lewis theories offer different perspectives on acid-base chemistry, they are not mutually exclusive. The Lewis theory encompasses the Brønsted-Lowry theory, providing a more comprehensive framework for understanding a wider range of acid-base reactions. Both theories are vital tools for chemists, offering valuable insights into reaction mechanisms, predicting reactivity, and designing new materials and catalysts. Understanding both theories and their applications is critical for a deep comprehension of acid-base chemistry and its vast implications across various chemical disciplines. The HSAB theory further refines our understanding by adding a predictive element to Lewis acid-base interactions. Mastering these theoretical frameworks is essential for anyone striving to excel in the field of chemistry.
Latest Posts
Latest Posts
-
What Is 1 And 2 3 As A Decimal
Mar 17, 2025
-
What Is The Electron Configuration Of Argon
Mar 17, 2025
-
Is Chlorine A Solid Liquid Or Gas
Mar 17, 2025
-
What Is The Lcm For 7 And 9
Mar 17, 2025
-
44 Is 25 Of What Number
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Lewis Acid And Base Vs Bronsted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
