Is Chlorine A Solid Liquid Or Gas
listenit
Mar 17, 2025 · 5 min read
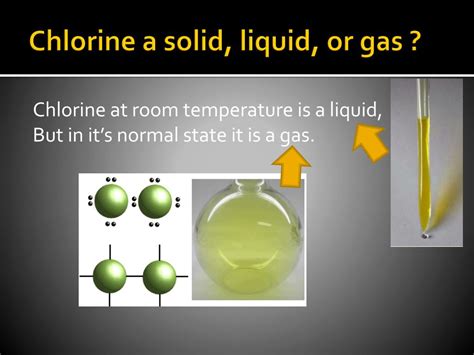
Table of Contents
Is Chlorine a Solid, Liquid, or Gas? Understanding Chlorine's States of Matter
Chlorine, a chemical element with the symbol Cl and atomic number 17, is a fascinating substance with properties that vary depending on its state. Understanding whether chlorine is a solid, liquid, or gas requires exploring its physical properties and how temperature and pressure influence its phase transitions. This comprehensive guide will delve into the details, clarifying the common misconceptions and providing a thorough understanding of chlorine's existence in different states of matter.
Chlorine's Natural State: A Gaseous Element
Under standard temperature and pressure (STP), defined as 0°C (273.15 K) and 1 atmosphere (atm), chlorine exists as a gas. This is its most common and naturally occurring form. Chlorine gas is a pale yellow-green, with a pungent and irritating odor readily detectable even at low concentrations. It's this gaseous form that often comes to mind when discussing chlorine's properties and applications, particularly in water treatment and industrial processes. The pungent smell serves as a crucial warning signal, as chlorine gas can be highly toxic and dangerous if inhaled in significant amounts.
The Significance of STP in Defining Chlorine's State
The definition of STP is critical in understanding the state of matter of any substance, including chlorine. A change in either temperature or pressure significantly alters the intermolecular forces within the chlorine molecules, leading to a change in its physical state. At STP, the kinetic energy of the chlorine molecules is sufficient to overcome the weak intermolecular forces, leading to their free movement characteristic of gases.
Chlorine's Phase Transitions: From Gas to Liquid to Solid
While chlorine's natural state is gaseous, it's capable of transitioning to both liquid and solid phases under specific conditions. These transitions involve changes in temperature and pressure that impact the kinetic energy of the chlorine molecules and the strength of the intermolecular forces.
Liquefaction of Chlorine Gas: Lowering the Temperature and/or Increasing the Pressure
To liquefy chlorine gas, one must either decrease the temperature below its boiling point or increase the pressure sufficiently to force the molecules closer together. Chlorine's boiling point at standard pressure is -34.04 °C (-29.07 °F). By cooling chlorine gas below this temperature, the kinetic energy of the molecules decreases, allowing the intermolecular forces to dominate, resulting in a transition to the liquid phase. Similarly, increasing the pressure reduces the volume available to the gas molecules, increasing the frequency of collisions and forcing them into closer proximity, eventually resulting in liquefaction. Liquid chlorine is a pale yellow-green liquid, denser than its gaseous form.
Solidification of Chlorine: Reaching Extremely Low Temperatures
To solidify chlorine, even lower temperatures are needed. The freezing point of chlorine is -101.5 °C (-150.7 °F). At temperatures below this threshold, the kinetic energy of the chlorine molecules becomes extremely low, and the intermolecular forces become strong enough to hold the molecules in a fixed, crystalline structure, forming solid chlorine. Solid chlorine is a pale yellow-green crystalline solid. It's important to note that achieving these low temperatures requires specialized equipment and conditions not encountered in everyday life.
Understanding Intermolecular Forces in Chlorine
The phase transitions of chlorine are governed by the interplay between its kinetic energy and intermolecular forces. While chlorine molecules are held together by strong covalent bonds within each molecule (Cl₂), the intermolecular forces between different chlorine molecules are comparatively weak. These weak forces, primarily van der Waals forces (London dispersion forces), are responsible for the relatively low boiling and freezing points of chlorine.
The Role of Van der Waals Forces
Van der Waals forces are weak, short-range attractive forces that exist between all molecules. In the case of chlorine, these forces increase in strength as the molecules are brought closer together, either by lowering the temperature or increasing the pressure. At low temperatures, the kinetic energy of the molecules decreases, allowing the weak van der Waals forces to become significant enough to hold the molecules together in the liquid and solid phases. Conversely, at higher temperatures, the kinetic energy overcomes these weak forces, resulting in the gaseous state.
Practical Applications of Chlorine in Different States
Chlorine's different states of matter find diverse applications across various industries.
Chlorine Gas: A Versatile Industrial Chemical
Chlorine gas is widely used in water purification to disinfect drinking water and swimming pools, eliminating harmful bacteria and pathogens. It's also employed in the production of numerous chemicals, including PVC (polyvinyl chloride) plastics, solvents, and pesticides. Other industrial applications include bleaching pulp and paper, and as a disinfectant in various industrial settings. The use of chlorine gas, however, necessitates stringent safety protocols due to its toxicity and hazardous nature.
Liquid Chlorine: Easier Handling and Transportation
Liquid chlorine is preferred for transportation and storage due to its higher density compared to the gaseous form. It allows for efficient transport in pressurized tanks, significantly reducing the volume and simplifying logistics. Many industrial processes utilize liquid chlorine as a safer and more convenient form of the element compared to handling the gas directly.
Solid Chlorine: A Less Common Application
Solid chlorine finds far fewer practical applications due to the difficulty in handling and maintaining its extremely low temperature. Specialized research and experimental settings might utilize solid chlorine, but its large-scale industrial applications are limited.
Safety Precautions when Handling Chlorine
Working with chlorine in any of its states requires adherence to strict safety protocols. Chlorine gas is highly toxic and irritating to the respiratory system, eyes, and skin. Exposure to high concentrations can be fatal. Proper ventilation and respiratory protection are crucial when handling chlorine gas. Liquid chlorine, while less immediately hazardous than the gas, presents risks associated with its pressure and potential for leaks. Specialized equipment and training are necessary for handling liquid chlorine safely.
Conclusion: Chlorine's State-Dependent Properties
In conclusion, chlorine's state—solid, liquid, or gas—is determined by temperature and pressure. While it commonly exists as a pale yellow-green gas under standard conditions, it can be liquefied by lowering the temperature or increasing the pressure and solidified at significantly lower temperatures. Understanding these phase transitions and the intermolecular forces governing them is critical for safe handling and effective application of this vital chemical element across a wide range of industries. The knowledge of its properties and behavior in different states ensures responsible usage and minimizes potential hazards. Remember, always prioritize safety when working with chlorine, irrespective of its state of matter.
Latest Posts
Latest Posts
-
170 Degrees To Radians In Terms Of Pi
Mar 17, 2025
-
Which Has More Inertia Tennis Ball Or Basketball
Mar 17, 2025
-
Is Air An Element Or Compound Or Mixture
Mar 17, 2025
-
What Percent Is 15 Of 75
Mar 17, 2025
-
Y 2 X 1 2 1 Graph
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Chlorine A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
