Lanthanides And Actinides On The Periodic Table
listenit
Mar 28, 2025 · 6 min read
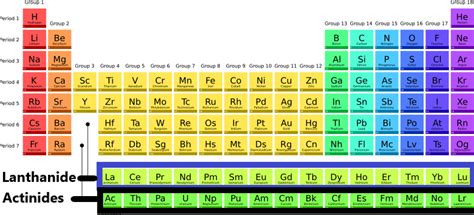
Table of Contents
Lanthanides and Actinides: The Inner Secrets of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While the main group elements and transition metals often take center stage, the lanthanides and actinides, nestled at the bottom of the table, represent a fascinating and often overlooked realm of chemical behavior. These two series, collectively known as the inner transition metals or f-block elements, possess unique characteristics that warrant a deeper exploration. This article delves into the intricacies of lanthanides and actinides, examining their electronic configurations, chemical properties, applications, and the challenges associated with their study.
Understanding the Electronic Configuration
The defining feature of the lanthanides and actinides is the progressive filling of the 4f and 5f orbitals, respectively. This contrasts with the transition metals where the d-orbitals are being filled. This inner orbital filling leads to many similarities in the chemical properties within each series, making separation and identification a significant challenge.
Lanthanides (4f Series):
The lanthanides, elements 57 (Lanthanum) to 71 (Lutetium), are characterized by the gradual filling of the 4f electron shell. While Lanthanum itself doesn't have a 4f electron, it's traditionally included due to its chemical similarities. The 4f orbitals are shielded by the filled 5s and 5p orbitals, resulting in a relatively weak influence on the chemical bonding characteristics. This shielding effect contributes to the similar chemical behaviors observed across the lanthanide series. This makes separation a very energy intensive and challenging process.
Actinides (5f Series):
The actinides, elements 89 (Actinium) to 103 (Lawrencium), mirror the lanthanides in their filling of the 5f orbitals. However, the actinides exhibit a greater range of oxidation states and more complex chemical behavior compared to their lanthanide counterparts. This is because the 5f orbitals are less effectively shielded than the 4f orbitals, leading to greater involvement in chemical bonding. Furthermore, the relativistic effects, becoming increasingly significant for heavier elements, play a crucial role in shaping the properties of the actinides. These effects alter electron energies and orbital sizes, impacting their reactivity and bonding.
Chemical Properties: Similarities and Differences
Despite their placement within distinct series, lanthanides and actinides share some common chemical characteristics. Both series predominantly exhibit +3 oxidation states, although other oxidation states are possible, especially within the actinide series. This prevalence of the +3 oxidation state is directly linked to the relative ease of removing three electrons from the outermost shells.
Lanthanides:
Lanthanides are relatively reactive metals, readily oxidizing in air to form oxides. Their +3 oxidation state dominates their chemistry. They form a variety of complexes with ligands, often displaying high coordination numbers. Their similar chemical properties make separation extremely difficult; techniques like ion-exchange chromatography are essential for purification.
Actinides:
Actinides present a more diverse range of chemical behaviors than lanthanides. While the +3 oxidation state is common, higher oxidation states like +4, +5, +6, and even +7 are observed, particularly in the early actinides. This variability in oxidation states contributes to their diverse range of chemical reactivity. Many actinides are radioactive, which adds significant complexity to their handling and study. Their reactivity also makes them environmentally significant, as their behavior needs careful consideration in nuclear waste management.
Applications: From Magnets to Nuclear Power
The unique properties of the lanthanides and actinides translate into a variety of applications across diverse fields. Their applications heavily reflect the characteristics of the individual element.
Lanthanide Applications:
- Magnets: Neodymium magnets (Nd₂Fe₁₄B), composed of a lanthanide (neodymium), iron, and boron, are incredibly powerful permanent magnets used in numerous applications, including hard disk drives, wind turbines, and electric motors. Their high magnetic strength is crucial for modern technological advancements.
- Catalysts: Certain lanthanides serve as catalysts in various industrial processes, such as petroleum cracking and the synthesis of various organic compounds. Their ability to change the rate of chemical reactions without being consumed themselves is invaluable.
- Lighting: Lanthanides are used in various lighting technologies, including fluorescent lamps and high-intensity discharge lamps. Their emission of characteristic colors when excited makes them essential components in producing specific light wavelengths.
- Medical Applications: Some lanthanides find applications in medical imaging and therapy. For example, gadolinium is employed as a contrast agent in magnetic resonance imaging (MRI).
Actinide Applications:
The primary application of actinides centers around their use in nuclear power generation and weaponry. The highly radioactive nature of these elements, while posing significant challenges in terms of safety and waste disposal, is also the source of their energy potential.
- Nuclear Fuel: Uranium-235 and Plutonium-239 are crucial fissile isotopes used in nuclear reactors to generate electricity. The controlled nuclear fission process releases vast amounts of energy.
- Nuclear Weapons: The same fissile isotopes utilized in nuclear reactors are also the basis of nuclear weapons. The uncontrolled chain reaction in a nuclear weapon releases an immense amount of destructive energy.
- Radioactive Tracers: Some actinides, due to their radioactivity, find limited applications as radioactive tracers in scientific research and medical diagnostics. Their decay patterns can be used to monitor specific processes.
Challenges and Considerations
Working with lanthanides and actinides presents several challenges:
Lanthanides:
- Separation: The similar chemical properties of lanthanides make their separation a complex and energy-intensive process. Advanced separation techniques, such as ion-exchange chromatography, are necessary to obtain pure samples.
- Toxicity: Some lanthanides can be toxic, requiring careful handling and disposal procedures.
Actinides:
- Radioactivity: The radioactivity of actinides necessitates stringent safety protocols and specialized handling equipment. Radiation protection is paramount.
- Nuclear Waste Management: The radioactive waste generated from nuclear power plants and weapons programs poses significant long-term environmental challenges. Safe and effective disposal methods are vital for protecting the environment and human health.
- Environmental Impact: The release of actinides into the environment can have severe consequences due to their radioactivity and chemical toxicity. Careful environmental monitoring and remediation are crucial.
Future Directions and Research
Research on lanthanides and actinides continues to advance, driving innovation across diverse fields. Areas of active investigation include:
- Improved Separation Techniques: Developing more efficient and cost-effective methods for separating lanthanides is crucial for expanding their applications.
- Novel Applications: Exploring new applications of lanthanides and actinides in areas such as advanced materials science, catalysis, and medicine is an ongoing endeavor.
- Nuclear Waste Remediation: Research focuses on developing safer and more sustainable methods for managing and remediating nuclear waste, including the immobilization and disposal of actinides.
- Fundamental Chemistry: A deeper understanding of the fundamental chemistry of these elements, particularly their electronic structure and reactivity, remains an active area of research.
Conclusion
The lanthanides and actinides, despite their often-overlooked position on the periodic table, are elements of profound importance. Their unique properties, stemming from the filling of the f-orbitals, have led to applications ranging from powerful magnets to nuclear power generation. However, their use also presents challenges, including separation difficulties for lanthanides and radioactive waste management for actinides. Continued research and development in this area are essential for maximizing the benefits of these elements while mitigating the associated risks and challenges. The fascinating world of the f-block elements promises continued scientific exploration and technological innovation for years to come.
Latest Posts
Latest Posts
-
How Many Electrons Are In The 4th Energy Level
Mar 31, 2025
-
How To Convert Molarity To Molality
Mar 31, 2025
-
How Much Is 1000 Feet In Miles
Mar 31, 2025
-
How Do You Write 20 As A Decimal
Mar 31, 2025
-
What Is The Densest Object In The Universe
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Lanthanides And Actinides On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
