Is Magnesium A Solid Liquid Or Gas
listenit
Mar 22, 2025 · 5 min read
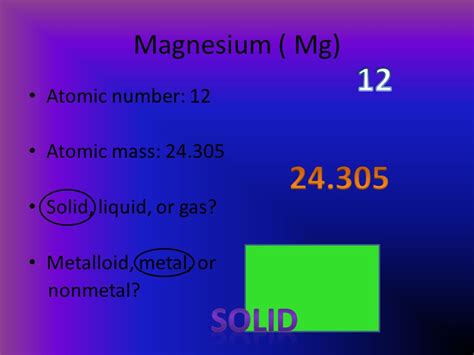
Table of Contents
Is Magnesium a Solid, Liquid, or Gas? Understanding Magnesium's Physical Properties
Magnesium, a vital element for both human health and numerous industrial applications, exists in a specific physical state under normal conditions. But what is that state? This comprehensive guide will delve deep into the physical properties of magnesium, clarifying its state of matter and exploring the conditions under which it might transition between solid, liquid, and gaseous phases.
Understanding States of Matter
Before diving into the specifics of magnesium, let's briefly review the three fundamental states of matter:
-
Solid: In a solid state, atoms or molecules are tightly packed together in a fixed arrangement. They possess strong intermolecular forces, resulting in a definite shape and volume. Solids resist changes in shape and volume.
-
Liquid: Liquids have weaker intermolecular forces than solids. Their atoms or molecules are more mobile and can move past each other, resulting in a definite volume but an indefinite shape. Liquids take the shape of their container.
-
Gas: Gases have the weakest intermolecular forces. Their atoms or molecules are widely dispersed and move randomly, resulting in an indefinite shape and volume. Gases expand to fill their container.
Magnesium at Room Temperature: A Solid State
Under standard temperature and pressure conditions (typically defined as 25°C and 1 atmosphere), magnesium exists as a solid. Its atoms are arranged in a closely packed hexagonal crystal structure. This structure contributes to magnesium's relatively high density and strength.
Key Characteristics of Solid Magnesium:
-
Silvery-white appearance: Magnesium possesses a characteristic lustrous, silvery-white metallic sheen. This is due to the interaction of light with its electrons.
-
Lightweight: Despite its strength, magnesium is surprisingly lightweight. Its low density makes it a valuable material in various applications where weight reduction is crucial.
-
Reactive metal: Magnesium is a moderately reactive metal, meaning it readily reacts with various substances such as oxygen and acids. This reactivity is responsible for some of its important uses, such as in combustion and metallurgical processes.
-
Good conductor of heat and electricity: Magnesium's metallic bonding allows for efficient conduction of heat and electricity, making it useful in electrical components and heat transfer systems.
-
Ductile and Malleable: These properties signify that magnesium can be readily drawn into wires (ductility) and hammered into sheets (malleability). This makes it relatively easy to work with in manufacturing processes.
Transitioning to Liquid Magnesium: Melting Point and Beyond
Magnesium transitions from its solid state to a liquid state at its melting point, which is approximately 650°C (1202°F). At temperatures above this point, the thermal energy overcomes the intermolecular forces holding the magnesium atoms in their fixed arrangement, causing them to become more mobile and flow like a liquid.
Properties of Liquid Magnesium:
-
High reactivity: Liquid magnesium retains its reactivity, even exhibiting increased reactivity due to the higher mobility of its atoms. Care must be taken when handling molten magnesium due to its potential to react vigorously with air and moisture.
-
Lower density than solid magnesium: Interestingly, the density of liquid magnesium is slightly lower than that of solid magnesium. This is atypical for many substances but results from the change in atomic arrangement as the material melts.
-
Applications in metallurgy: Liquid magnesium plays a crucial role in various metallurgical processes, including alloy production and casting. It's often used as an alloying agent to enhance the properties of other metals.
-
High temperature processing challenges: Working with liquid magnesium requires specialized equipment and techniques due to its high melting point and reactivity at elevated temperatures.
Transitioning to Gaseous Magnesium: Boiling Point and Beyond
Further heating of liquid magnesium to its boiling point (approximately 1090°C (1994°F)) causes it to transition into a gaseous state. At this point, the intermolecular forces are completely overcome, and the magnesium atoms move freely and independently, exhibiting characteristics of a gas.
Properties of Gaseous Magnesium:
-
Highly reactive: Gaseous magnesium is even more reactive than liquid magnesium due to the increased freedom of movement of the atoms and increased exposure to other reactants.
-
Low density: Gaseous magnesium possesses an extremely low density, making it less dense than air.
-
Specialized applications: While less common than its solid or liquid forms, gaseous magnesium finds niche applications in specialized chemical processes and research settings. For example, it may be involved in certain types of chemical vapor deposition (CVD) processes used to create thin films.
Factors Affecting Magnesium's State: Pressure and Impurities
While temperature plays the most significant role in determining magnesium's state of matter, pressure can also exert an influence, though this is typically less pronounced than the effect of temperature. Increased pressure generally favors the denser solid phase.
The presence of impurities can also affect magnesium's melting and boiling points slightly. The addition of other elements can alter the intermolecular forces and thus the temperatures at which phase transitions occur.
Real-World Applications of Magnesium in Different States
The applications of magnesium vary widely depending on its state:
-
Solid Magnesium: Used extensively in automotive parts (wheels, engine components), aerospace components (aircraft parts), electronics (battery casings), and biomedical devices (implants). Its lightweight, strength, and biocompatibility make it highly desirable in these sectors.
-
Liquid Magnesium: Primarily used in the production of magnesium alloys and in casting processes to create various components. Its reactivity in the molten state is both a challenge and an opportunity in various metallurgical applications.
-
Gaseous Magnesium: Although less common, gaseous magnesium plays a role in specialized chemical reactions, thin film deposition processes, and research studies exploring magnesium's behavior in different environments.
Conclusion: Magnesium's Versatile Nature
Magnesium's physical state is fundamentally determined by temperature and, to a lesser extent, pressure and the presence of impurities. Under normal conditions, it exists as a solid, a strong and lightweight material with numerous practical applications. However, understanding its melting and boiling points and the properties of its liquid and gaseous states is crucial for its effective use in various industrial processes and advanced technologies. The unique characteristics of magnesium in each of its states highlight its versatility and importance in diverse fields. Further research into magnesium's behavior under extreme conditions continues to reveal its multifaceted properties and expand its potential applications.
Latest Posts
Latest Posts
-
Find Equation Of Plane Through Point And Parallel To Plane
Mar 24, 2025
-
What Is The Charge Of Cl
Mar 24, 2025
-
What Is Prime Factorization Of 63
Mar 24, 2025
-
Least Common Multiple 14 And 21
Mar 24, 2025
-
What Is The Lcm Of 8 12
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Magnesium A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
