Is Ice Melting Endothermic Or Exothermic
listenit
Mar 14, 2025 · 6 min read
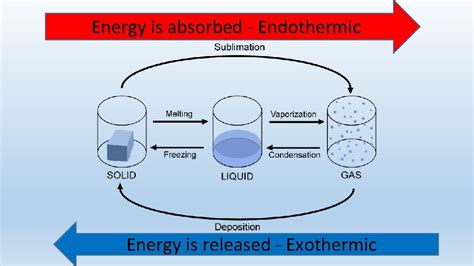
Table of Contents
Is Ice Melting Endothermic or Exothermic? A Deep Dive into Thermodynamics
The question of whether ice melting is endothermic or exothermic is a fundamental concept in thermodynamics, often encountered in chemistry and physics classes. While the answer seems straightforward, a deeper understanding requires exploring the nuances of energy transfer and phase transitions. This article will delve into the intricacies of this process, explaining not only the answer but also the underlying principles and practical implications.
Understanding Endothermic and Exothermic Processes
Before we tackle the specifics of ice melting, let's establish a clear definition of endothermic and exothermic processes. These terms describe the energy exchange between a system and its surroundings during a process.
-
Endothermic processes: These processes absorb heat energy from their surroundings. The system's energy increases, leading to a decrease in the surrounding temperature. Think of it like a sponge soaking up water; the sponge (system) gains energy (water), and the environment (surroundings) loses energy.
-
Exothermic processes: These processes release heat energy to their surroundings. The system's energy decreases, leading to an increase in the surrounding temperature. Imagine a burning candle; the candle (system) loses energy (heat and light), and the environment (surroundings) gains energy (heat and light).
The Phase Transition: From Solid to Liquid
Ice melting is a phase transition, specifically a change from the solid state (ice) to the liquid state (water). During this transition, the water molecules in the ice crystal lattice gain enough kinetic energy to overcome the intermolecular forces holding them in a rigid structure. This allows them to move more freely, transitioning from the ordered arrangement of a solid to the less ordered arrangement of a liquid.
Why Ice Melting is Endothermic
The key to understanding why ice melting is endothermic lies in the energy required to break those intermolecular forces. The energy needed to overcome these attractive forces is absorbed from the surroundings. Therefore, the melting process absorbs heat energy, making it an endothermic process.
The Role of Heat Capacity and Latent Heat of Fusion
Two important thermodynamic concepts play crucial roles in understanding the energy changes involved in ice melting:
-
Heat Capacity: This refers to the amount of heat energy required to raise the temperature of a substance by a certain amount. Water has a relatively high heat capacity, meaning it takes a significant amount of energy to change its temperature.
-
Latent Heat of Fusion: This is the amount of heat energy required to change a substance from a solid to a liquid at its melting point without changing its temperature. For water, the latent heat of fusion is approximately 334 kJ/kg. This means that 334 kilojoules of energy are needed to melt one kilogram of ice at 0°C. This energy goes entirely into breaking the hydrogen bonds holding the water molecules in the ice lattice, not into raising the temperature.
This latent heat explains why the temperature of ice remains at 0°C while it's melting, even though it's absorbing heat. All the absorbed energy is used to break the bonds, not to increase the kinetic energy (and thus temperature) of the water molecules.
Practical Applications and Real-World Examples
The endothermic nature of ice melting has numerous practical applications in various fields:
-
Cooling Systems: Ice is commonly used as a coolant because its melting absorbs heat from its surroundings, thus lowering the temperature. This principle is utilized in refrigerators, air conditioning systems, and even in simple applications like cooling drinks. The melting ice absorbs heat from the beverage, keeping it cool.
-
Biological Systems: The endothermic nature of ice melting plays a critical role in various biological processes. For instance, the melting of ice in the spring helps regulate the temperature of ecosystems and allows for the thawing of frozen ground, facilitating plant growth.
-
Industrial Processes: Controlled melting of ice is used in various industrial processes, such as in the production of certain materials and in the processing of food products.
-
Weather Phenomena: The melting of ice and snow significantly impacts weather patterns. The energy absorbed during melting influences air temperature, humidity, and cloud formation. The massive energy absorption associated with melting vast ice sheets and glaciers contribute to complex climate change dynamics.
Misconceptions and Clarifications
Some common misconceptions surrounding ice melting need clarification:
-
Ice melting 'produces' cold: Ice melting doesn't produce cold; it absorbs heat. The feeling of coldness arises from the heat transfer from your hand (or whatever is in contact with the ice) to the melting ice. The ice is not actively generating cold; it's passively absorbing heat.
-
Temperature change during melting: The temperature of the ice-water mixture remains constant at 0°C (32°F) during the melting process. This is because all the absorbed heat is used to overcome the intermolecular forces, not to raise the temperature. Only after all the ice has melted will the temperature of the resulting water begin to rise if more heat is added.
Deeper Dive into Thermodynamics: Gibbs Free Energy and Equilibrium
For a more advanced understanding, let's look at the concept of Gibbs Free Energy (ΔG). Gibbs Free Energy is a thermodynamic potential that can be used to predict the spontaneity of a process at constant temperature and pressure. The equation is:
ΔG = ΔH - TΔS
Where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content)
- T is the temperature in Kelvin
- ΔS is the change in entropy (disorder)
In the case of ice melting, ΔH is positive (endothermic), and ΔS is also positive (the liquid state is more disordered than the solid state). At temperatures above 0°C, the TΔS term becomes larger than ΔH, making ΔG negative. This negative ΔG indicates that the melting of ice is spontaneous above 0°C. Below 0°C, the reverse is true, and freezing is spontaneous. At 0°C, the system is at equilibrium, and the rate of melting equals the rate of freezing.
Conclusion: Ice Melting: A Clear Case of Endothermy
In conclusion, ice melting is unequivocally an endothermic process. The absorption of heat energy is essential to overcome the intermolecular forces holding the water molecules in the ice crystal lattice, allowing the transition to the liquid phase. Understanding this fundamental thermodynamic principle is crucial in various scientific disciplines and has far-reaching practical applications in everyday life and industrial processes. The concepts of latent heat of fusion, Gibbs Free Energy, and the relationship between enthalpy and entropy provide a comprehensive explanation for the endothermic nature of this ubiquitous phase transition. This process, seemingly simple, highlights the complexities and elegance of thermodynamics.
Latest Posts
Latest Posts
-
Find Points Where Tangent Line Is Horizontal
Mar 15, 2025
-
3 X 2 4 X 2
Mar 15, 2025
-
Is Boiling Water A Chemical Change Or Physical Change
Mar 15, 2025
-
6 32 To The Power Of 2
Mar 15, 2025
-
What Is The Least Common Multiple Of 10 And 4
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Ice Melting Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
