Is Boiling Water A Chemical Change Or Physical Change
listenit
Mar 15, 2025 · 6 min read
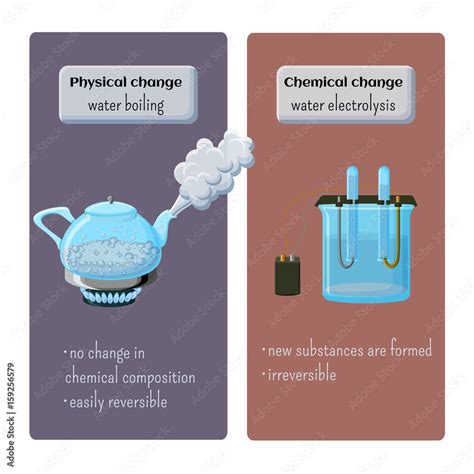
Table of Contents
Is Boiling Water a Chemical Change or a Physical Change? A Deep Dive
The question of whether boiling water represents a chemical or physical change is a fundamental one in chemistry, often encountered early in scientific education. While the answer might seem straightforward at first glance, a deeper exploration reveals a fascinating interplay of concepts and subtly nuanced distinctions. This comprehensive article will delve into the intricacies of this question, exploring the definitions of chemical and physical changes, examining the process of boiling water in detail, and addressing common misconceptions. We'll also touch upon related concepts to provide a complete understanding of this often-debated topic.
Understanding Chemical vs. Physical Changes
Before we tackle the specific case of boiling water, let's establish clear definitions of chemical and physical changes. This foundation is crucial for accurately classifying any transformation matter undergoes.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The fundamental building blocks – the molecules – remain the same. Think of cutting a piece of paper, melting an ice cube, or dissolving sugar in water. In each case, the substance is modified in some way, but its chemical identity persists. The changes are generally reversible; you can often recover the original substance. Key characteristics of physical changes include:
- No new substance is formed: The chemical formula remains unchanged.
- Changes are often reversible: The original substance can usually be recovered.
- Usually involve changes in physical properties: These include changes in state (solid, liquid, gas), shape, size, or density.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. This rearrangement typically involves the breaking and forming of chemical bonds. Examples include burning wood, rusting iron, or cooking an egg. These changes are often irreversible, meaning you can't easily get back the original substances. Key indicators of a chemical change include:
- Formation of a new substance: The chemical formula changes.
- Changes are often irreversible: The original substance cannot easily be recovered.
- Often accompanied by observable changes: These can include changes in color, temperature, odor, or the production of gas or precipitate.
Analyzing the Boiling of Water
Now, let's apply these definitions to the boiling of water. When water boils, it transitions from its liquid state to its gaseous state (steam). This is a phase transition, a change in physical state. But does this change the chemical composition of water?
The Molecular Perspective
Water, in its chemical form, is represented by the formula H₂O, meaning each molecule is composed of two hydrogen atoms bonded to one oxygen atom. When water boils, the molecules gain enough kinetic energy to overcome the intermolecular forces holding them together in the liquid state. They escape into the gaseous phase as steam. Importantly, the individual H₂O molecules themselves remain intact. There is no breaking or forming of chemical bonds between the hydrogen and oxygen atoms within the water molecules.
Observable Changes vs. Chemical Changes
While boiling water shows observable changes—a transition from liquid to gas, the production of bubbles, an increase in temperature—none of these indicate a fundamental alteration in the chemical makeup of the water. The steam produced is still H₂O; it's just in a different physical state. You can condense the steam back into liquid water, demonstrating the reversibility of the process.
Addressing Common Misconceptions
It's crucial to dispel some common misconceptions surrounding boiling water:
-
Bubbles are not a sign of a chemical change: The bubbles in boiling water are primarily composed of water vapor. While dissolved gases might also be released, the formation of bubbles themselves isn't indicative of a chemical reaction.
-
Temperature increase doesn't imply a chemical change: The increase in temperature during boiling simply reflects the increased kinetic energy of the water molecules, allowing them to overcome intermolecular forces. It doesn't involve the breaking or forming of chemical bonds within the water molecule.
-
Electrolysis is a different process: Electrolysis of water is a separate chemical process, where an electric current is used to decompose water into its constituent elements, hydrogen (H₂) and oxygen (O₂). This is fundamentally different from simply boiling water.
Boiling Water: A Definitive Conclusion
Based on our analysis, the boiling of water is undeniably a physical change. The chemical composition of the water remains unchanged. The transformation from liquid to gas involves only a change in the physical state of the water, driven by an increase in kinetic energy. The molecules remain the same; only their arrangement and interaction change. The process is reversible, and the original substance (water) can be readily recovered.
Beyond Boiling: Exploring Related Phase Transitions
Understanding the boiling of water provides a crucial foundation for understanding other phase transitions. Let's briefly explore some related concepts:
Melting and Freezing: Solid to Liquid and Vice Versa
Similar to boiling, melting and freezing are physical changes. Ice melting into water and water freezing into ice both involve changes in the arrangement of water molecules but not their chemical composition. These processes are also reversible.
Sublimation and Deposition: Solid to Gas and Gas to Solid
Sublimation is the transition of a substance directly from the solid to the gaseous phase without passing through the liquid phase (e.g., dry ice). Deposition is the reverse process, where a gas transitions directly to a solid. Both are physical changes, maintaining the chemical integrity of the substance.
Evaporation: A Gradual Phase Transition
Evaporation is another physical change where a liquid transitions to a gas, but it occurs at temperatures below the boiling point. This process is slower than boiling and occurs at the surface of the liquid. Like boiling, evaporation doesn't alter the chemical makeup of the substance.
Practical Applications and Real-World Examples
Understanding the distinction between physical and chemical changes is essential in many fields:
-
Cooking: Many cooking processes involve both physical and chemical changes. Boiling vegetables is primarily a physical change, while baking a cake involves numerous complex chemical reactions.
-
Industrial Processes: Chemical engineers carefully control physical and chemical changes in various industrial settings, from refining petroleum to manufacturing pharmaceuticals.
-
Environmental Science: Understanding phase transitions is crucial for studying weather patterns, water cycles, and climate change.
-
Materials Science: The development of new materials often involves manipulating physical and chemical properties through controlled changes.
Conclusion: A Foundation for Further Learning
The simple act of boiling water provides a compelling example of a physical change. By thoroughly examining this process, we've solidified our understanding of the fundamental differences between physical and chemical changes. This knowledge forms a solid foundation for exploring more complex chemical and physical phenomena. The ability to distinguish between these types of changes is a cornerstone of scientific literacy and crucial for understanding the world around us. Continued learning and exploration in chemistry will reveal even more fascinating intricacies within the realm of matter and its transformations.
Latest Posts
Latest Posts
-
Is Water A Good Leaving Group
Mar 17, 2025
-
480 Cm Equals How Many M
Mar 17, 2025
-
What Is 8 In Fraction Form
Mar 17, 2025
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Chemical Change Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
