Is Water A Good Leaving Group
listenit
Mar 17, 2025 · 5 min read
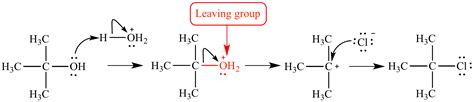
Table of Contents
Is Water a Good Leaving Group? A Deep Dive into Leaving Group Ability
Water's role in organic chemistry reactions is multifaceted. It acts as a solvent, a reactant, and sometimes, surprisingly, as a leaving group. Understanding whether water qualifies as a good leaving group requires a nuanced look at its properties and the context of the reaction. This article will explore the intricacies of water's leaving group ability, examining the factors that influence its effectiveness and providing examples to illustrate its behavior in different reaction scenarios.
Understanding Leaving Groups
Before diving into water's specifics, let's establish a fundamental understanding of leaving groups. A leaving group is an atom or group of atoms that departs from a molecule, taking with it a pair of electrons. The better a leaving group, the more readily it departs. This ability is directly related to its stability as an independent species.
Several factors contribute to the stability, and hence the leaving group ability, of a species:
-
Weak basicity: Good leaving groups are weak bases. A weak base is less likely to react with the remaining molecule after leaving, thereby favoring the forward reaction. Strong bases, in contrast, tend to readily react with the resulting carbocation or other electrophilic species, causing the reaction to reverse.
-
Resonance stabilization: If the leaving group can be stabilized through resonance, it will be a better leaving group. Resonance delocalizes the negative charge, making the leaving group more stable.
-
High electronegativity: Electronegative atoms are better able to stabilize the negative charge they acquire when they depart.
-
Size: Larger leaving groups are generally better because the negative charge is more dispersed.
Water as a Leaving Group: A Complex Picture
Water (H₂O) possesses some characteristics of a good leaving group, but it falls short in others. Let's analyze its strengths and weaknesses:
Weaknesses of Water as a Leaving Group:
-
Moderate basicity: While not a strong base, water is a stronger base than many other common leaving groups like halides (Cl⁻, Br⁻, I⁻) or tosylates. This means it's more likely to react with the resulting carbocation or electrophile, hindering the reaction progress.
-
Poor resonance stabilization: Water does not exhibit significant resonance stabilization. The negative charge acquired upon leaving remains localized on the oxygen atom, making it less stable.
-
Neutral charge: Although it's not directly related to basicity, the neutral charge of water in its initial state requires extra activation energy for ionization. This ionization step creates the charged leaving group (hydroxide, OH⁻) which is a poorer leaving group than water itself.
Strengths of Water as a Leaving Group:
-
Abundance and availability: Water is readily available as a solvent, which is a significant practical advantage in many reactions.
-
Protic solvent effect: Water's protic nature facilitates the reactions where it acts as a leaving group by stabilizing intermediates and transition states. This can partially compensate for its relatively weak leaving group ability.
Reaction Conditions Favoring Water as a Leaving Group
Water's effectiveness as a leaving group is highly dependent on the reaction conditions. Several factors can significantly influence its ability to depart:
-
Strong acids: The presence of a strong acid protonates the hydroxyl group in water, converting it into a better leaving group, H₂O. This protonation reduces the basicity of the oxygen atom and facilitates its departure. Reactions involving dehydration of alcohols often utilize strong acid catalysts for this very reason.
-
High temperature: Increasing the temperature increases the kinetic energy of the molecules, providing the activation energy required for the water molecule to leave.
-
Specific reaction mechanisms: Certain reaction mechanisms are more conducive to water acting as a leaving group than others. For instance, E1 and SN1 reactions that involve carbocation intermediates are more tolerant of poorer leaving groups such as water compared to SN2 reactions.
Examples of Water as a Leaving Group
Several reaction types illustrate water's role as a leaving group, although often indirectly and with the assistance of other factors:
Acid-catalyzed dehydration of alcohols:
This classic reaction exemplifies water's role as a leaving group. Under acidic conditions, an alcohol undergoes protonation of the hydroxyl group, followed by loss of water to form a carbocation intermediate. Subsequent deprotonation leads to the formation of an alkene. The strong acid protonates the -OH group, converting it into a better leaving group (H₂O).
Hydrolysis reactions under acidic conditions:
In some hydrolysis reactions, water can act as a nucleophile and a leaving group simultaneously. For instance, the hydrolysis of esters under acidic conditions involves the addition of water followed by the elimination of an alcohol molecule (acting as a leaving group). Water itself can also become a leaving group later in the reaction.
Formation of Ethers from Alcohols (Acid-catalyzed):
This reaction involves the protonation of the hydroxyl group of one alcohol molecule, facilitating the elimination of water and subsequent nucleophilic attack of another alcohol molecule. Once again, acid catalysis is crucial for the protonation of hydroxyl groups, aiding in the departure of water as a leaving group.
Conclusion: Is Water a Good Leaving Group? A Contextual Answer
The question of whether water is a good leaving group is not a simple yes or no answer. It depends heavily on the specific reaction conditions and the reaction mechanism. Compared to halide ions, sulfonate esters, or other common leaving groups, water is generally considered a poor leaving group. However, under certain conditions, particularly in the presence of strong acids or at elevated temperatures, water can function as a leaving group, albeit often requiring significant activation energy and careful control of the reaction environment. Its presence in a given reaction should always be considered in the context of the reaction pathway, the stability of the potential leaving group, and the reaction conditions employed.
Therefore, understanding the nuances of water's behavior as a leaving group requires a thorough analysis of the reaction in its entirety. While it's not an ideal leaving group, its abundance and involvement in many reaction mechanisms make it a crucial component to consider in understanding organic reaction pathways. The examples provided illustrate how water's role as a leaving group can be significantly enhanced through protonation, which makes it a more potent leaving group in reality. Its role is frequently intertwined with the reaction conditions and the overall mechanistic pathway, a factor crucial to understanding the effectiveness of any leaving group.
Latest Posts
Latest Posts
-
Is Oil Polar Or Non Polar
May 13, 2025
-
What Is The Derivative Of 3e X
May 13, 2025
-
Solubility Of Hydrogen Chloride In Water
May 13, 2025
-
Solve For The Value Of R
May 13, 2025
-
Which Of The Following Are The Basic Units Of Matter
May 13, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.