Is Ice Melting A Chemical Reaction
listenit
Mar 31, 2025 · 5 min read
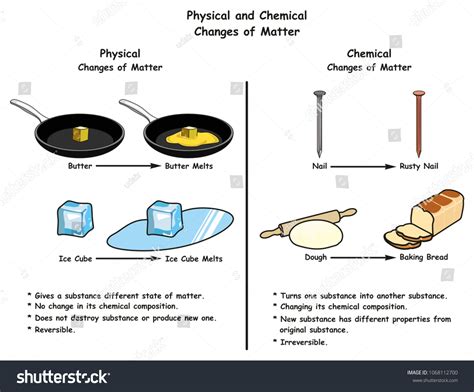
Table of Contents
Is Ice Melting a Chemical Reaction? Understanding the Physics of Phase Transitions
The question of whether ice melting is a chemical reaction often sparks debate. While it involves a significant transformation, it's crucial to understand that ice melting is a physical change, not a chemical reaction. This distinction hinges on the fundamental definition of each process: chemical reactions involve the breaking and forming of chemical bonds, while physical changes alter the form or appearance of a substance without changing its chemical composition. Let's delve deeper into this fascinating phenomenon, exploring the underlying principles and clarifying common misconceptions.
The Difference Between Physical and Chemical Changes
Before we definitively answer the question regarding ice melting, let's solidify our understanding of the core concepts.
Chemical Reactions: Breaking and Making Bonds
A chemical reaction involves a rearrangement of atoms, resulting in the formation of new substances with different properties. This rearrangement occurs through the breaking and forming of chemical bonds—the strong forces that hold atoms together in molecules. Chemical reactions are often accompanied by observable changes such as color change, gas evolution, precipitate formation, or a significant temperature change. Examples include burning wood (combustion), rusting iron (oxidation), and baking a cake (complex series of reactions).
Physical Changes: No Bond Alteration
In contrast, a physical change alters the appearance or form of a substance without changing its chemical composition. No new substances are created; the chemical bonds within the molecules remain intact. Examples of physical changes include melting ice, boiling water, dissolving sugar in water, or bending a wire. These changes are often reversible, meaning the original substance can be recovered.
The Physics of Ice Melting: A Phase Transition
Ice melting is a classic example of a phase transition. Phase transitions are physical processes where a substance changes from one state of matter (solid, liquid, gas, or plasma) to another. These transitions are driven by changes in temperature and/or pressure.
From Solid to Liquid: A Change in Molecular Arrangement
When ice melts, it transitions from the solid phase (ice) to the liquid phase (water). This transition doesn't involve breaking the chemical bonds between the hydrogen and oxygen atoms within the water molecules (H₂O). Instead, it involves a change in the arrangement and interaction of these molecules.
In ice, water molecules are tightly packed in a highly ordered crystalline structure held together by relatively weak intermolecular forces (hydrogen bonds). These bonds constrain the molecules' movement, resulting in a rigid structure.
As heat is applied, the kinetic energy of the water molecules increases. This increased energy overcomes the intermolecular forces holding the molecules in their fixed positions within the crystal lattice. The structure begins to break down, and the molecules gain greater freedom of movement, transitioning into the liquid phase. The molecules are still H₂O, but their arrangement is less ordered and more fluid.
The Role of Temperature and Heat Energy
Temperature is a measure of the average kinetic energy of the molecules. To melt ice, sufficient heat energy must be supplied to overcome the intermolecular forces holding the water molecules in the crystalline structure. This energy is absorbed by the ice, causing the molecules to vibrate more vigorously and eventually break free from their fixed positions. This explains why ice melts at 0°C (32°F) at standard atmospheric pressure – this is the temperature at which the kinetic energy of the molecules is sufficient to overcome the intermolecular forces.
Why Ice Melting Isn't a Chemical Reaction: A Deeper Look
Let's examine several key characteristics that confirm ice melting as a physical change:
- No new substance is formed: The chemical composition remains unchanged. The melted ice is still water (H₂O); no new molecules are created.
- The process is reversible: By lowering the temperature below 0°C, the liquid water can be re-frozen into ice. This reversibility is a hallmark of physical changes.
- No chemical bonds are broken or formed: The strong covalent bonds within the water molecules remain intact throughout the melting process. Only the weaker intermolecular hydrogen bonds are affected.
- The change is primarily driven by a change in kinetic energy: The absorption of heat increases the kinetic energy of the water molecules, allowing them to overcome intermolecular forces, not to break covalent bonds.
Common Misconceptions about Ice Melting
Despite the clear scientific explanation, some misconceptions persist:
-
Misconception 1: A change of state always means a chemical reaction: This is false. Phase transitions, like melting, boiling, and freezing, are physical changes. They involve changes in the state of matter but not the chemical composition.
-
Misconception 2: The appearance of liquid water is a sign of a chemical reaction: The change in appearance from solid ice to liquid water is a consequence of the altered molecular arrangement, not the formation of new substances.
-
Misconception 3: Energy changes indicate a chemical reaction: While energy is absorbed during melting, the energy change is associated with the breaking of intermolecular forces, not chemical bonds. Many physical changes involve energy changes.
The Importance of Understanding Phase Transitions
Understanding phase transitions, such as ice melting, is crucial in various fields:
- Climate science: Melting ice contributes significantly to sea-level rise and impacts global climate patterns.
- Material science: The properties of many materials are heavily influenced by their phase.
- Engineering: Phase transitions are important in various engineering processes, such as refrigeration and material processing.
- Chemistry: Understanding phase transitions is fundamental to understanding the behavior of matter under different conditions.
Conclusion: Ice Melting – A Physical Process
In conclusion, ice melting is unequivocally a physical change, not a chemical reaction. It involves a phase transition from solid to liquid, driven by the increase in kinetic energy of water molecules overcoming intermolecular forces. The chemical composition of water remains unchanged throughout the process, and the transition is reversible. Understanding this distinction is critical for appreciating the fundamental principles of matter and its behavior under various conditions. The misconception that it is a chemical reaction stems from a misunderstanding of the differences between physical and chemical changes, highlighting the need for clear and concise scientific explanations. The process's significance extends far beyond a simple observation, touching upon critical scientific and environmental concerns.
Latest Posts
Latest Posts
-
If S Glyceraldehyde Has A Specific Rotation Of
Apr 02, 2025
-
How Many Neutrons Does Molybdenum Have
Apr 02, 2025
-
How Monomers Are Related To Polymers
Apr 02, 2025
-
Does Gas Take The Shape Of Its Container
Apr 02, 2025
-
2 5 To The Power Of 3
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Ice Melting A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
