Is H2co3 An Acid Or Base
listenit
Mar 22, 2025 · 6 min read
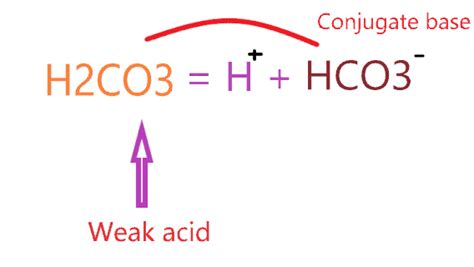
Table of Contents
Is H₂CO₃ an Acid or a Base? Understanding Carbonic Acid's Properties
Carbonic acid (H₂CO₃) is a weak acid, not a base. This seemingly simple statement belies a complex reality involving chemical equilibrium, dissociation constants, and its significant role in various biological and environmental processes. Understanding its acidic nature requires delving into its chemical structure, behavior in aqueous solutions, and its impact on pH. This comprehensive guide will explore these aspects in detail, addressing common misconceptions and clarifying the fundamental properties of carbonic acid.
The Chemical Structure and Nature of H₂CO₃
Carbonic acid's chemical formula, H₂CO₃, suggests the presence of two acidic protons (H⁺ ions). These protons are attached to oxygen atoms, which are in turn bonded to a central carbon atom also double-bonded to another oxygen atom. This structure is crucial in determining its acidic behavior. The O-H bonds are relatively polar, making the hydrogen atoms susceptible to dissociation in the presence of a base or in aqueous solution. This dissociation is what gives carbonic acid its acidic character. The structure facilitates the release of protons, leading to a decrease in pH. It's important to remember that the actual concentration of H₂CO₃ in solution is relatively low compared to its conjugate base, bicarbonate (HCO₃⁻). This is because H₂CO₃ readily forms from the reaction of carbon dioxide (CO₂) with water.
The Formation of Carbonic Acid
H₂CO₃ isn't typically prepared directly. Instead, it forms in an equilibrium reaction between carbon dioxide (CO₂) and water (H₂O):
CO₂(aq) + H₂O(l) ⇌ H₂CO₃(aq)
This equilibrium lies far to the left, meaning that only a small fraction of dissolved CO₂ actually converts into H₂CO₃. Most dissolved CO₂ remains as CO₂ molecules. Nevertheless, the small amount of H₂CO₃ formed is sufficient to significantly impact the acidity of the solution. This reaction's equilibrium constant is relatively small, signifying that the formation of carbonic acid is not strongly favored.
Dissociation of Carbonic Acid and Acid Strength
The key to understanding carbonic acid's acidic nature lies in its dissociation in water. A strong acid, like hydrochloric acid (HCl), completely dissociates into its ions in water, releasing all its protons. However, H₂CO₃ is a weak acid, meaning it only partially dissociates. This means that a significant portion of H₂CO₃ molecules remain undissociated in solution, creating an equilibrium between undissociated acid and its conjugate base and hydrogen ions.
The first dissociation step is:
H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
This step has an acid dissociation constant (Ka₁) of approximately 4.3 x 10⁻⁷ at 25°C. The Ka value indicates the strength of an acid: lower Ka values signify weaker acids. The relatively small Ka₁ for carbonic acid confirms its weak acid character.
The second dissociation step is:
HCO₃⁻(aq) ⇌ H⁺(aq) + CO₃²⁻(aq)
This step has a much smaller Ka₂ (about 4.8 x 10⁻¹¹ at 25°C) than Ka₁, indicating that bicarbonate (HCO₃⁻) is a significantly weaker acid than carbonic acid itself. This sequential dissociation explains the buffering capacity of the carbonic acid-bicarbonate system, which is crucial for maintaining blood pH in humans and other biological systems.
The Role of Carbonic Acid in Biological Systems
Carbonic acid's weak acidity plays a vital role in maintaining the pH balance in various biological systems. The carbonic acid-bicarbonate buffer system is one of the most important buffering systems in human blood. It helps to resist changes in blood pH, preventing potentially life-threatening acidosis (low blood pH) or alkalosis (high blood pH). This buffer system operates effectively because the concentrations of H₂CO₃ and HCO₃⁻ are carefully regulated within the body.
The lungs and kidneys play significant roles in this regulation. The lungs control the concentration of CO₂, which in turn affects the concentration of H₂CO₃. The kidneys control the concentration of bicarbonate (HCO₃⁻) by either reabsorbing it or excreting it into the urine. This intricate interplay between the respiratory and renal systems ensures the efficient maintenance of blood pH. Disruptions to this system can lead to severe health consequences, highlighting the crucial role of carbonic acid in biological homeostasis.
Furthermore, carbonic acid and its related species play significant roles in other biological processes, including:
- Photosynthesis: In plants, carbon dioxide is incorporated into organic molecules during photosynthesis. While not directly involving H₂CO₃, the dissolved CO₂ serves as the starting material.
- Shell formation in marine organisms: Many marine organisms use bicarbonate ions (HCO₃⁻) to build their shells and exoskeletons, demonstrating the importance of the carbonic acid system in marine ecosystems.
Carbonic Acid and Environmental Impact
The carbonic acid system has significant implications for environmental chemistry and climate change. The absorption of atmospheric CO₂ by the oceans leads to the formation of carbonic acid, causing ocean acidification. Increased atmospheric CO₂ concentrations lead to higher levels of dissolved CO₂ in the oceans. This, in turn, increases the concentration of H₂CO₃, lowering the ocean's pH. This acidification has harmful effects on marine ecosystems, particularly on organisms that build shells or exoskeletons from calcium carbonate (CaCO₃), such as corals and shellfish. The increased acidity makes it more difficult for these organisms to build and maintain their structures, potentially leading to population declines and ecosystem disruption.
The impact of ocean acidification is a complex issue, involving multiple interconnected factors beyond simply the increased concentration of H₂CO₃. However, understanding the fundamental chemistry of carbonic acid and its role in the carbon cycle is essential for comprehending the environmental consequences of increasing atmospheric CO₂ levels.
Addressing Common Misconceptions
It's important to address some common misconceptions surrounding carbonic acid:
- Confusion with bicarbonate: While bicarbonate (HCO₃⁻) is closely related to carbonic acid and plays a crucial role in buffering systems, it is not the same thing. Bicarbonate is the conjugate base of carbonic acid.
- Overestimation of H₂CO₃ concentration: It's crucial to remember that the actual concentration of H₂CO₃ in solution is relatively low because the equilibrium strongly favors dissolved CO₂. Most of the dissolved carbon dioxide exists as CO₂ molecules rather than H₂CO₃.
- Mistaking it for a strong acid: Because it releases protons, some might mistakenly categorize it as a strong acid. However, its partial dissociation and low Ka values clearly indicate its weak acid nature.
Conclusion: H₂CO₃ as a Weak Acid and Its Importance
In conclusion, carbonic acid (H₂CO₃) is definitively a weak acid, not a base. Its weak acidity, coupled with its role in the carbonic acid-bicarbonate buffer system, makes it incredibly significant in biological systems and environmental chemistry. Understanding its chemical structure, dissociation, and equilibrium reactions is crucial for grasping its importance in maintaining pH balance in blood, regulating marine ecosystems, and understanding the effects of climate change on the oceans. The low concentration of H₂CO₃ in solution compared to its dissolved CO₂ precursor often leads to misconceptions, which this article has sought to address comprehensively. The information provided here offers a thorough exploration of carbonic acid's properties and its profound impact on life and the environment.
Latest Posts
Latest Posts
-
60 Of What Number Is 12
Mar 24, 2025
-
Effects Of Acid Rain In Germany
Mar 24, 2025
-
Prokaryotic Cells Do Not Have What
Mar 24, 2025
-
Which Is The Most Abundant Gas In Earths Atmosphere
Mar 24, 2025
-
How Many Carbon Atoms Are There In A Propane Molecule
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is H2co3 An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
