How Many Carbon Atoms Are There In A Propane Molecule
listenit
Mar 24, 2025 · 5 min read
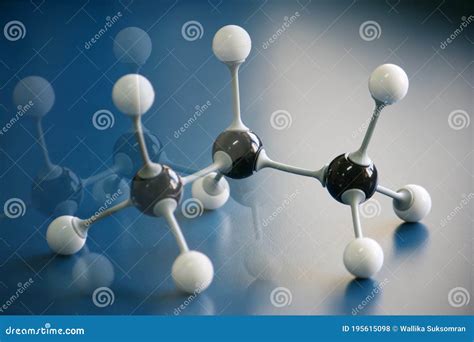
Table of Contents
How Many Carbon Atoms Are There in a Propane Molecule? A Deep Dive into Propane's Structure and Properties
Propane, a ubiquitous fuel source in homes and industries, holds a deceptively simple yet fascinating molecular structure. Understanding this structure is crucial to grasping its properties and applications. This comprehensive guide delves into the fundamental question: how many carbon atoms are there in a propane molecule? We'll explore this seemingly straightforward question in detail, examining propane's chemical formula, its three-dimensional structure, and the implications of its carbon atom count for its behavior.
Deciphering the Chemical Formula: C3H8
The chemical formula for propane is C₃H₈. This concise notation immediately reveals the answer to our central question: propane contains three carbon atoms. The subscript "3" next to the "C" (representing carbon) explicitly states the number of carbon atoms present in a single propane molecule. The "H₈" indicates the presence of eight hydrogen atoms, completing the molecule's structure.
Understanding Chemical Formulas: A Quick Primer
Chemical formulas are shorthand representations of molecules, providing a quick overview of their elemental composition. Understanding how to interpret them is vital for anyone working with chemicals. The elements are represented by their chemical symbols (e.g., C for carbon, H for hydrogen, O for oxygen). Subscripts indicate the number of atoms of each element present in a single molecule. For example:
- H₂O: Water contains two hydrogen atoms and one oxygen atom.
- CO₂: Carbon dioxide contains one carbon atom and two oxygen atoms.
- C₆H₁₂O₆: Glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Visualizing the Propane Molecule: Structure and Bonding
Knowing that propane has three carbon atoms is only half the story. Understanding its three-dimensional structure is key to appreciating its properties. The three carbon atoms in propane are arranged in a chain, connected by single covalent bonds. Each carbon atom forms four bonds, a consequence of its valence electron configuration.
Covalent Bonds: The Glue Holding Propane Together
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In propane, each carbon atom shares electrons with its neighboring carbon atoms and hydrogen atoms. The carbon-carbon single bonds (C-C) are strong and relatively stable, while the carbon-hydrogen single bonds (C-H) are also strong. These bonds determine the overall shape and reactivity of the propane molecule.
The Linear Arrangement: Not Entirely Straight!
While often depicted as a straight line in simplified diagrams, the propane molecule isn't perfectly linear. The bond angles between the atoms are approximately 109.5 degrees, resulting in a slightly zig-zag arrangement. This tetrahedral geometry around each carbon atom is a consequence of the repulsion between the electron pairs involved in the covalent bonds.
The Significance of Three Carbon Atoms: Impact on Properties
The presence of three carbon atoms in propane directly influences its physical and chemical properties. Let's explore some of these key characteristics:
Boiling Point and Phase at Room Temperature
Propane's relatively low molecular weight (due to its three carbons and eight hydrogens) results in weak intermolecular forces. This translates to a low boiling point (-42°C or -44°F), making it a gas at room temperature and atmospheric pressure.
Flammability and Energy Density
Propane is highly flammable, a property directly related to the presence of carbon-hydrogen bonds. These bonds store significant energy, releasing it as heat and light when propane undergoes combustion. The relatively high energy density of propane makes it an efficient fuel source.
Solubility and Polarity
Propane is a nonpolar molecule, meaning it has no significant separation of charge within its structure. This nonpolar nature makes it largely insoluble in water (a polar solvent) but readily soluble in nonpolar solvents such as other hydrocarbons.
Comparing Propane to Other Alkanes: Exploring the Homologous Series
Propane belongs to a homologous series of hydrocarbons known as alkanes. Alkanes are characterized by carbon-carbon single bonds and are saturated hydrocarbons, meaning they have the maximum number of hydrogen atoms bonded to their carbon atoms. The number of carbon atoms distinguishes members of this series.
- Methane (CH₄): One carbon atom.
- Ethane (C₂H₆): Two carbon atoms.
- Propane (C₃H₈): Three carbon atoms.
- Butane (C₄H₁₀): Four carbon atoms.
- Pentane (C₅H₁₂): Five carbon atoms.
And so on, with each additional carbon atom leading to changes in the molecule's properties. As the number of carbon atoms increases, the boiling point increases, and the viscosity of the alkane also increases.
Propane's Applications: From Fuel to Refrigerant
The unique properties of propane, stemming from its three-carbon structure, make it highly versatile:
-
Fuel Source: Propane is widely used as a fuel for heating homes, powering barbeque grills, and fueling vehicles (autogas). Its high energy density and relatively clean combustion make it a desirable energy source.
-
Refrigerant: Propane's ability to absorb heat makes it suitable as a refrigerant in certain applications. It's a more environmentally friendly alternative to some traditional refrigerants with harmful environmental impacts.
-
Petrochemical Feedstock: Propane serves as a feedstock for the production of various petrochemicals, including propylene, which is used in the manufacture of plastics and other materials.
Conclusion: The Significance of Three
The seemingly simple answer – three – to the question, "How many carbon atoms are there in a propane molecule?" belies the profound implications this number has on propane's structure, properties, and applications. Understanding propane's molecular structure is fundamental to appreciating its role in our daily lives, from heating our homes to powering our vehicles. The relationship between the number of carbon atoms, molecular structure, and resulting properties is a key concept in chemistry, showcasing the power of understanding the fundamental building blocks of matter. This detailed exploration of propane provides a strong foundation for further exploration of organic chemistry and the fascinating world of hydrocarbons.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 21 And 28
Mar 25, 2025
-
Rank The Following Atoms According To Their Size
Mar 25, 2025
-
How Do You Find The Y Intercept Of Two Points
Mar 25, 2025
-
How Many Radians Are In A Revolution
Mar 25, 2025
-
3 4 To The Power Of 2
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Carbon Atoms Are There In A Propane Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
