Is Gas Evaporating A Chemical Change
listenit
Mar 23, 2025 · 5 min read
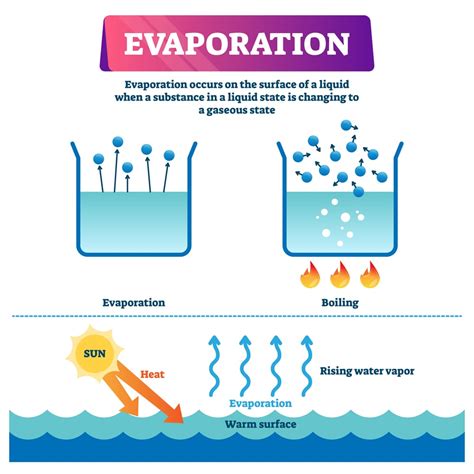
Table of Contents
Is Gas Evaporating a Chemical Change? Understanding the Difference Between Physical and Chemical Changes
The question of whether gas evaporating constitutes a chemical change or a physical change is a fundamental concept in chemistry. While seemingly simple, a clear understanding requires delving into the definitions of chemical and physical changes and applying them to the specific process of evaporation. This article will explore the nuances of evaporation, examining its characteristics and comparing it to true chemical reactions to definitively answer the question.
Defining Chemical and Physical Changes
Before we tackle the evaporation question, let's establish a solid foundation by defining the key terms:
Physical Change: A physical change alters the form or appearance of a substance without changing its chemical composition. The molecules themselves remain unchanged; only their arrangement or state of matter might be altered. Examples include melting ice, dissolving sugar in water, or cutting a piece of wood. These changes are typically reversible.
Chemical Change: A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into new substances with different chemical properties. This transformation involves the breaking and forming of chemical bonds, leading to a change in molecular structure. Examples include burning wood, rusting iron, or baking a cake. These changes are often irreversible.
The Process of Evaporation: A Deep Dive
Evaporation is the process by which a liquid transitions to a gaseous state below its boiling point. This happens when molecules at the surface of the liquid gain enough kinetic energy to overcome the intermolecular forces holding them together and escape into the surrounding atmosphere.
Key Characteristics of Evaporation:
- Temperature Dependence: Evaporation occurs more rapidly at higher temperatures because molecules possess greater kinetic energy.
- Surface Area: A larger surface area exposes more liquid molecules to the atmosphere, increasing the rate of evaporation.
- Air Movement: Increased air movement removes evaporated molecules from the vicinity of the liquid surface, preventing condensation and promoting further evaporation.
- Humidity: High humidity (high concentration of water vapor in the air) slows down evaporation because the air is already saturated with water molecules.
- Nature of the Liquid: Different liquids have different intermolecular forces and vapor pressures, influencing their evaporation rates. For example, ethanol evaporates much faster than water.
Analyzing Evaporation through the Lens of Chemical Change Criteria
Let's examine evaporation based on the defining criteria of a chemical change:
-
Formation of new substances: Evaporation does not produce new chemical substances. The water molecules in gaseous state are still H₂O molecules; only their physical state has changed. There is no change in their chemical composition or molecular structure.
-
Breaking and formation of chemical bonds: No chemical bonds are broken or formed during evaporation. The intermolecular forces (hydrogen bonds in the case of water) between water molecules are weakened and overcome, but the covalent bonds within the water molecule itself remain intact.
-
Irreversibility: Evaporation is reversible. The water vapor can condense back into liquid water under appropriate conditions (cooling, increased pressure). Chemical changes are often, though not always, irreversible.
-
Energy Changes: While evaporation does involve an energy change (the liquid absorbs heat to overcome intermolecular forces), this energy change is primarily a change in the kinetic energy of the molecules, not a rearrangement of chemical bonds. Exothermic and endothermic reactions involve changes in potential energy due to breaking and forming chemical bonds.
Contrasting Evaporation with Chemical Reactions
To further solidify the understanding, let's contrast evaporation with a clear example of a chemical reaction: combustion.
When wood burns (combustion), it reacts with oxygen in the air, forming new substances like carbon dioxide (CO₂) and water (H₂O). This involves the breaking of chemical bonds in the wood and oxygen molecules and the formation of new bonds in the CO₂ and H₂O molecules. This reaction is irreversible and produces significant energy changes (heat and light).
The key difference lies in the fundamental transformation of matter. In combustion, new substances with distinct chemical properties are formed. In evaporation, only the physical state of the substance changes.
Conclusion: Evaporation is a Physical Change
Based on the detailed analysis above, it is clear that evaporation is a physical change, not a chemical change. No new substances are formed, no chemical bonds are broken or formed, and the process is reversible. The only change is the transition of a substance from the liquid to the gaseous state due to changes in the kinetic energy of its molecules. While energy is involved, this energy is used to overcome intermolecular forces, not break intramolecular bonds, a crucial difference. The understanding of this distinction is critical for mastering fundamental chemistry concepts.
Further Exploring Related Concepts
Understanding evaporation leads to a deeper understanding of related concepts like:
- Boiling: Boiling is a special case of evaporation where the vapor pressure of the liquid equals the external pressure, allowing vaporization to occur throughout the liquid, not just at the surface. It's still a physical change.
- Sublimation: Sublimation is the direct transition of a substance from the solid to the gaseous phase, bypassing the liquid phase. Again, it's a physical change as no new substance is formed.
- Condensation: The opposite of evaporation, condensation is the transition from gas to liquid. This is also a physical change.
By carefully examining the processes and analyzing them against the criteria of chemical and physical changes, we can confidently classify these phenomena, establishing a solid understanding of the fundamental principles of chemistry. The distinction between physical and chemical changes is crucial for understanding numerous scientific phenomena and processes.
Latest Posts
Latest Posts
-
Nuclear Symbol For Gallium With 40 Neutrons
Mar 24, 2025
-
What Is 95 As A Fraction
Mar 24, 2025
-
Number Of Valence Electrons For Sodium
Mar 24, 2025
-
What Is The Molar Mass Of Helium
Mar 24, 2025
-
What Role Do Decomposers Play In The Carbon Cycle
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Gas Evaporating A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
