What Is The Molar Mass Of Helium
listenit
Mar 24, 2025 · 6 min read
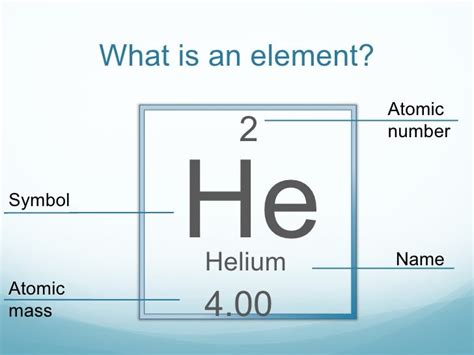
Table of Contents
What is the Molar Mass of Helium? A Deep Dive into Atomic Weight and Applications
Helium, the second lightest element on the periodic table, is a fascinating and incredibly useful substance with a wide range of applications. Understanding its properties, particularly its molar mass, is crucial to comprehending its behavior and diverse uses. This comprehensive article delves into the concept of molar mass, specifically focusing on helium, exploring its calculation, significance, and relevance across various scientific and technological fields.
Understanding Molar Mass: The Foundation
Before we delve into the specifics of helium's molar mass, let's establish a firm understanding of what molar mass represents. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities, whether they are atoms, molecules, ions, or other specified particles. Essentially, it's a way to relate the microscopic world of atoms and molecules to the macroscopic world of grams and kilograms that we experience daily.
The molar mass is numerically equivalent to the atomic weight (for elements) or molecular weight (for compounds) expressed in grams per mole (g/mol). This equivalence stems from the definition of the mole and the carefully calibrated atomic mass unit (amu). One amu is defined as 1/12th the mass of a carbon-12 atom, providing a consistent and reliable standard for measuring atomic and molecular masses.
Calculating the Molar Mass of Helium
Helium (He) is an element, meaning it exists as individual atoms, not molecules. Its atomic number is 2, indicating it possesses two protons in its nucleus. The most abundant isotope of helium is helium-4 (⁴He), which contains two protons and two neutrons. A small amount of helium-3 (³He) also exists, with one less neutron. However, for practical purposes, the molar mass of helium is typically calculated based on the weighted average of its naturally occurring isotopes.
The standard atomic weight of helium is approximately 4.0026 amu. This is a weighted average reflecting the relative abundance of helium-4 and helium-3 in the Earth's atmosphere. Since the molar mass is numerically equivalent to the atomic weight, the molar mass of helium is approximately 4.0026 g/mol. This means that one mole of helium atoms has a mass of 4.0026 grams.
Factors Influencing the Precision of Molar Mass
It is important to note that the reported value of helium's molar mass (and the atomic weight of other elements) can vary slightly depending on the source and the method used for measurement. These slight variations stem from:
- Isotopic Abundance: The precise relative abundance of helium isotopes can differ slightly depending on the source of the helium sample (e.g., terrestrial helium vs. helium from radioactive decay).
- Measurement Techniques: Different mass spectrometry techniques used to determine isotopic abundances can yield slightly different results.
- Rounding: Published values are often rounded to a certain number of significant figures.
However, the difference in molar mass values between various sources is typically negligible for most practical applications. The value of 4.0026 g/mol provides a sufficient level of accuracy for the vast majority of calculations.
The Significance of Helium's Molar Mass
Knowing the molar mass of helium is crucial for numerous applications in chemistry, physics, and engineering. It is fundamental for:
-
Stoichiometric Calculations: In chemical reactions involving helium, the molar mass is essential for converting between the mass of helium and the number of moles, allowing accurate calculations of reactant and product quantities. This is vital in various chemical processes, including those in materials science and industrial chemistry.
-
Gas Law Calculations: Helium's molar mass plays a crucial role in gas law calculations, particularly the Ideal Gas Law (PV=nRT). Using the molar mass, we can determine the number of moles (n) of helium in a given volume (V) at a specific temperature (T) and pressure (P), allowing us to predict its behavior under varying conditions.
-
Density Calculations: The density of helium gas can be calculated using its molar mass and the Ideal Gas Law. Understanding helium's density is critical for applications like aerostatics (balloons and airships) where buoyancy is essential.
-
Aerospace Engineering: In aerospace engineering, accurate knowledge of helium’s molar mass is vital for designing and operating spacecraft and other aerospace systems. Calculations involving helium's properties are critical for tasks like propellant management and leak detection.
-
Medical Applications: Helium’s low density and inertness make it important in medical applications such as MRI (magnetic resonance imaging). Understanding its molar mass contributes to accurate calculations involving helium mixtures used in these procedures.
Applications Leveraging Helium's Unique Properties
Helium's unique properties, stemming directly from its low atomic weight and inertness, have led to a wide array of applications across various sectors:
1. Cryogenics: Cooling to Extremely Low Temperatures
Helium's exceptionally low boiling point (-268.93 °C) makes it an indispensable cryogenic refrigerant. Its molar mass contributes to its low boiling point and subsequently its ability to cool superconducting magnets in MRI machines and other scientific instruments, enabling advanced research in fields like particle physics and material science. Understanding its molar mass allows precise control of its temperature and pressure in these cryogenic systems.
2. Leak Detection: Finding the Invisible Escapes
Helium's small atomic size and inertness make it an ideal tracer gas for leak detection in high-vacuum systems and other sealed environments. Helium leak detectors can sensitively pinpoint even tiny leaks, ensuring the integrity of crucial systems in various industries, from semiconductor manufacturing to aerospace engineering. Precise calculations involving helium’s molar mass support accurate leak detection analysis.
3. Welding and Shielding: Protecting the Process
Helium's inertness provides a protective atmosphere for welding processes, preventing oxidation and contamination. Its low density enables it to efficiently shield the weld zone, leading to superior weld quality. Knowing its molar mass is crucial for controlling the flow rate and pressure of helium used in welding applications.
4. Scientific Instrumentation: Enabling Advanced Research
Helium's unique properties are vital in various scientific instruments like mass spectrometers, gas chromatographs, and laser technology. Its use in these instruments often requires precise calculations involving its molar mass for calibration and data interpretation.
5. Breathing Mixtures: For Deep Sea Diving
Helium is often used in specialized breathing mixtures for deep-sea diving, due to its low density and inertness. These mixtures minimize the risk of decompression sickness and other diving-related health issues. Precise control over the composition of these mixtures requires an accurate understanding of helium's molar mass.
Conclusion: The Importance of Precision and Understanding
The molar mass of helium, approximately 4.0026 g/mol, is not simply a numerical value; it's a fundamental property that underpins its wide range of applications. Understanding this value allows for accurate calculations in diverse fields, from stoichiometry and gas laws to the design of advanced technologies. As we continue to explore new applications for helium, accurate knowledge of its molar mass will remain crucial for driving innovation and scientific advancement. The precision of this value, while seemingly small, significantly impacts the accuracy and reliability of various scientific and technological processes. Further research into isotopic abundances and measurement techniques will continue to refine our understanding of helium's molar mass, ensuring its continued significance in the years to come.
Latest Posts
Latest Posts
-
Which Balanced Equation Represents A Redox Reaction
Mar 28, 2025
-
Solve This Equation 2s S 12 132
Mar 28, 2025
-
What Is The Least Common Multiple Of 14 And 10
Mar 28, 2025
-
Is Water Condensing Endothermic Or Exothermic
Mar 28, 2025
-
Sum Of Exterior Angles Of A Heptagon
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
