Is Corrosion A Physical Or Chemical Property
listenit
Mar 23, 2025 · 6 min read
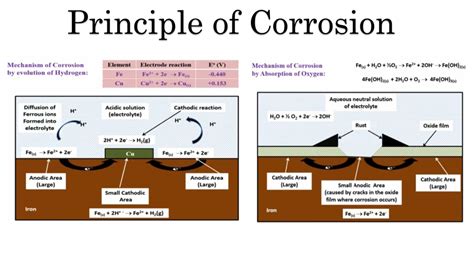
Table of Contents
Is Corrosion a Physical or Chemical Property? A Deep Dive
Corrosion, the gradual deterioration of a material due to a reaction with its environment, is a question that often sparks debate: is it a physical or chemical change? The answer, as with many nuanced scientific concepts, isn't a simple "yes" or "no." While exhibiting characteristics of both, corrosion is fundamentally a chemical process. This article will delve into the intricacies of corrosion, explaining why it's classified as a chemical change while also acknowledging the physical manifestations it presents.
Understanding the Difference: Physical vs. Chemical Changes
Before we dissect the nature of corrosion, let's establish a clear understanding of the distinction between physical and chemical changes.
Physical changes alter the form or appearance of a substance without changing its chemical composition. Examples include melting ice (water changes state but remains H₂O), dissolving sugar in water (sugar particles disperse but remain sugar molecules), or cutting a piece of wood (the wood's shape changes, but it remains wood). These changes are often reversible.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Examples include burning wood (wood reacts with oxygen to produce ash, carbon dioxide, and water), rusting iron (iron reacts with oxygen and water to form iron oxide), or baking a cake (ingredients react to form a new substance with different properties). These changes are typically irreversible.
The Chemical Nature of Corrosion
Corrosion, at its core, is a chemical reaction involving the oxidation of a material. This typically occurs when a metal reacts with its environment, most commonly with oxygen and water. The process involves the transfer of electrons from the metal atoms to the oxidant (usually oxygen), leading to the formation of metal oxides or other compounds.
Key Chemical Processes in Corrosion:
- Oxidation: The loss of electrons by the metal atoms. This is the fundamental chemical process driving corrosion.
- Reduction: The gain of electrons by the oxidant (e.g., oxygen). This process occurs simultaneously with oxidation, ensuring the overall balance of charge.
- Electrochemical Reactions: Corrosion often involves electrochemical reactions, where electron transfer occurs through an electrolyte (e.g., water containing dissolved ions). This creates an electrochemical cell, with different regions of the metal acting as anodes (where oxidation occurs) and cathodes (where reduction occurs).
- Formation of Corrosion Products: The chemical reaction produces new compounds, such as metal oxides, hydroxides, sulfates, or chlorides. These corrosion products are often different in color, texture, and properties from the original metal. For example, rust (iron oxide) is distinctly different from iron.
Examples illustrating the chemical nature of corrosion:
- Rusting of Iron: Iron (Fe) reacts with oxygen (O₂) and water (H₂O) to form hydrated iron(III) oxide (Fe₂O₃·xH₂O), commonly known as rust. This is a clear chemical transformation, producing a new substance with vastly different properties than iron. The chemical equation is a complex one, but the overall reaction can be simplified as: 4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s) which then dehydrates to form Fe₂O₃.
- Corrosion of Aluminum: Aluminum (Al) readily reacts with oxygen to form a thin, protective layer of aluminum oxide (Al₂O₃). While seemingly a simple reaction, the formation of this oxide layer is a chemical process that prevents further corrosion (passivation). The chemical reaction is: 4Al(s) + 3O₂(g) → 2Al₂O₃(s)
- Tarnishing of Silver: Silver (Ag) reacts with sulfur compounds in the air (e.g., hydrogen sulfide, H₂S) to form silver sulfide (Ag₂S), a dark tarnish. This is a chemical reaction leading to a change in the appearance and properties of the silver. The chemical reaction is: 2Ag(s) + H₂S(g) → Ag₂S(s) + H₂(g)
Physical Manifestations of Corrosion
Although fundamentally a chemical process, corrosion exhibits several physical manifestations:
- Changes in Appearance: Corrosion often results in visible changes to the material's appearance, including discoloration, pitting, cracking, scaling, or the formation of deposits. These are physical changes, but they are consequences of the underlying chemical reactions.
- Changes in Dimensions: Corrosion can alter the dimensions of the material, leading to thinning, weakening, or even complete disintegration. Again, this is a physical manifestation of the chemical attack.
- Changes in Mechanical Properties: The mechanical properties of the corroded material, such as strength, hardness, and ductility, are often degraded. This is a direct result of the chemical alteration of the material's structure.
- Changes in Mass: The mass of the material may increase (due to the addition of corrosion products) or decrease (due to the loss of material). This is a measurable physical change.
These physical changes are important indicators of corrosion, but they don't define the process itself. They are simply observable effects of the underlying chemical reactions.
Factors Influencing Corrosion Rate
Several factors influence the rate at which corrosion occurs:
- Type of Metal: Different metals have different tendencies to corrode. The reactivity of a metal, as determined by its position in the electrochemical series, is a crucial factor. Noble metals like gold and platinum are highly resistant to corrosion, while reactive metals like sodium and potassium corrode readily.
- Environment: The surrounding environment plays a critical role. Factors like humidity, temperature, pH, presence of corrosive substances (acids, salts, etc.), and oxygen concentration significantly affect corrosion rates.
- Surface Area: A larger surface area exposed to the environment typically results in faster corrosion.
- Presence of Inhibitors: Certain substances can slow down or prevent corrosion. These are called corrosion inhibitors.
- Stress and Strain: Internal stresses or external strains within the metal can accelerate corrosion.
Corrosion Prevention and Mitigation
Given the economic and safety implications of corrosion, preventing or mitigating it is crucial. Strategies include:
- Material Selection: Choosing corrosion-resistant materials (e.g., stainless steel, aluminum alloys) for specific applications.
- Protective Coatings: Applying coatings such as paints, polymers, or metallic coatings (e.g., galvanization) to protect the metal from the environment.
- Corrosion Inhibitors: Adding chemical inhibitors to the environment to slow down the corrosion process.
- Cathodic Protection: Using sacrificial anodes or impressed current cathodic protection to protect a metal structure from corrosion.
- Design Modifications: Designing structures to minimize the accumulation of moisture or corrosive substances.
Conclusion: Corrosion – A Chemical Change with Physical Manifestations
In conclusion, although corrosion exhibits many physical manifestations like changes in appearance, dimensions, and mechanical properties, its essence lies in the chemical reactions that underpin the process. The oxidation of the metal, the transfer of electrons, and the formation of new chemical compounds are the hallmarks of corrosion. The physical changes are merely observable consequences of this fundamental chemical transformation. Understanding this distinction is crucial for developing effective strategies for corrosion prevention and mitigation, ensuring the longevity and safety of various materials and structures. This knowledge underpins much of the materials science and engineering fields, driving innovation in the design and protection of everything from bridges and pipelines to automotive components and microelectronics. The ongoing research into corrosion mechanisms and prevention methods continues to be a vital area of scientific exploration with far-reaching implications across numerous industries.
Latest Posts
Latest Posts
-
Which Of The Following Elements Has The Highest Electronegativity
Mar 25, 2025
-
Calculate The Ph At Equivalence Point
Mar 25, 2025
-
Derivative Of 4 Square Root X
Mar 25, 2025
-
What Is A Equivalent Fraction For 5 8
Mar 25, 2025
-
How Many Water Molecules In A Drop
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Corrosion A Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
