Calculate The Ph At Equivalence Point
listenit
Mar 25, 2025 · 5 min read
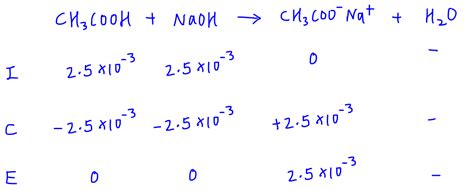
Table of Contents
Calculating the pH at the Equivalence Point of a Titration
Determining the pH at the equivalence point of a titration is crucial for understanding the titration curve and selecting appropriate indicators. The equivalence point represents the stoichiometric point where the moles of acid and base are equal, resulting in complete neutralization. However, the pH at this point isn't always neutral (pH 7). It depends on the strength of the acid and base involved. This comprehensive guide explores how to calculate the pH at the equivalence point for various titration types.
Understanding the Equivalence Point
The equivalence point signifies the completion of a neutralization reaction between an acid and a base. At this point, the moles of H⁺ ions from the acid precisely equal the moles of OH⁻ ions from the base. It's important to distinguish the equivalence point from the endpoint, which is the point where the indicator changes color, signifying the approximate completion of the reaction. Ideally, the endpoint should closely match the equivalence point.
Calculating pH at the Equivalence Point: Different Scenarios
The calculation method varies depending on the nature of the acid and base involved:
1. Strong Acid - Strong Base Titration
This is the simplest case. Since both the acid and base completely dissociate, the resulting salt solution at the equivalence point is neutral. Therefore, the pH at the equivalence point is 7.
Example: Titration of HCl (strong acid) with NaOH (strong base). The net ionic equation is:
H⁺(aq) + OH⁻(aq) → H₂O(l)
At the equivalence point, all H⁺ and OH⁻ ions react to form water, leaving only neutral water molecules. Hence, pH = 7.
2. Weak Acid - Strong Base Titration
This is more complex. The salt formed at the equivalence point is the conjugate base of the weak acid, which undergoes hydrolysis (reacts with water). This hydrolysis produces OH⁻ ions, resulting in a pH greater than 7 (basic).
Calculation Steps:
-
Determine the concentration of the conjugate base: At the equivalence point, the moles of the weak acid equal the moles of the strong base added. Use this information to calculate the concentration of the conjugate base formed.
-
Calculate the Kb of the conjugate base: The Kb (base dissociation constant) is related to the Ka (acid dissociation constant) of the weak acid by the equation: Kb = Kw / Ka, where Kw is the ion product constant of water (1.0 x 10⁻¹⁴ at 25°C).
-
Use the ICE table (Initial, Change, Equilibrium) to calculate the [OH⁻]: Set up an ICE table for the hydrolysis reaction of the conjugate base:
A⁻(aq) + H₂O(l) ⇌ HA(aq) + OH⁻(aq)
Use the Kb value and the initial concentration of the conjugate base to calculate the equilibrium concentration of OH⁻.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Example: Titration of acetic acid (weak acid) with NaOH (strong base). The conjugate base formed is acetate ion (CH₃COO⁻), which undergoes hydrolysis. You'd follow the steps above to determine the pH at the equivalence point.
3. Weak Base - Strong Acid Titration
This is analogous to the weak acid - strong base titration, but the resulting solution at the equivalence point will be acidic (pH less than 7). The salt formed is the conjugate acid of the weak base, which undergoes hydrolysis, producing H⁺ ions.
Calculation Steps:
The steps are similar to those for a weak acid - strong base titration, but instead of Kb, you will use the Ka of the conjugate acid: Ka = Kw / Kb. You'll set up an ICE table for the hydrolysis of the conjugate acid and calculate the [H⁺], then the pH.
4. Polyprotic Acid - Strong Base Titration
Polyprotic acids have multiple ionizable protons. For example, phosphoric acid (H₃PO₄) has three. The titration curve will show multiple equivalence points, one for each proton. Calculating the pH at each equivalence point requires considering the equilibrium of each deprotonation step. The calculations are more involved and often require iterative methods or approximation techniques. For instance, you might need to use the Henderson-Hasselbalch equation for the buffer regions between equivalence points.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point:
-
Concentration of the acid and base: Higher concentrations generally lead to a steeper titration curve and a sharper change in pH near the equivalence point.
-
Temperature: The Kw value changes with temperature, affecting the pH calculations.
-
Ionic strength: High ionic strength can affect the activity coefficients of ions, impacting the equilibrium calculations.
Choosing the Right Indicator
The choice of indicator for a titration depends heavily on the pH at the equivalence point. The indicator's pH range should encompass the equivalence point to ensure accurate determination of the endpoint. For example:
-
Strong acid-strong base titrations: Phenolphthalein or bromothymol blue are often suitable because the equivalence point is at pH 7.
-
Weak acid-strong base titrations: Phenolphthalein is a common choice because the equivalence point is above pH 7.
-
Weak base-strong acid titrations: Methyl orange or methyl red are often used because the equivalence point is below pH 7.
Advanced Considerations and Approximation Techniques
For complex scenarios involving polyprotic acids or very dilute solutions, simplifying assumptions may not be valid. More rigorous calculations may be necessary using iterative methods or computer software. Approximation techniques, such as neglecting the autoionization of water when dealing with relatively strong acids or bases, can be employed to simplify the calculations. However, always carefully evaluate the validity of these approximations.
Conclusion
Calculating the pH at the equivalence point is a fundamental concept in acid-base titrations. The procedure is straightforward for strong acid-strong base titrations but becomes more complex for weak acid-strong base or weak base-strong acid titrations. Understanding the underlying principles and employing appropriate calculation methods are crucial for accurately determining the equivalence point and selecting suitable indicators. Remember to consider the specific characteristics of the acid and base involved, and don’t hesitate to use appropriate approximation techniques when they are justified by the circumstances. Precise pH calculations at the equivalence point offer valuable insights into the stoichiometry and equilibrium properties of acid-base reactions.
Latest Posts
Latest Posts
-
Is Amu The Same As G Mol
Mar 25, 2025
-
Determining The Ksp Of Calcium Hydroxide
Mar 25, 2025
-
What Is The Difference Between Current And Static Electricity
Mar 25, 2025
-
Greatest Common Factor Of 15 And 60
Mar 25, 2025
-
How Many Electrons Are Shared In A Covalent Bond
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Ph At Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
