How Many Electrons Are Shared In A Covalent Bond
listenit
Mar 25, 2025 · 6 min read
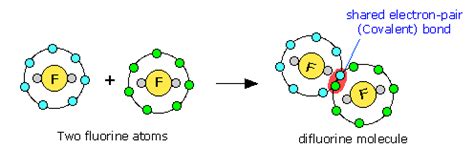
Table of Contents
How Many Electrons Are Shared in a Covalent Bond? A Deep Dive
Understanding the nature of chemical bonds is fundamental to grasping the behavior of matter. Among the various types of chemical bonds, covalent bonds stand out for their crucial role in the formation of a vast array of molecules, from simple diatomic gases to complex biomolecules. This article delves into the intricacies of covalent bonds, focusing specifically on the number of electrons shared between atoms and the factors influencing this sharing.
What is a Covalent Bond?
A covalent bond is a chemical bond formed when two atoms share one or more pairs of electrons. Unlike ionic bonds, which involve the transfer of electrons from one atom to another, covalent bonds arise from the mutual attraction of atoms for shared electrons. This sharing allows each atom to achieve a more stable electron configuration, typically resembling that of a noble gas with a full outer electron shell (octet rule).
The strength of a covalent bond depends on the degree of overlap between the atomic orbitals involved in the sharing. Greater overlap leads to a stronger bond. The shared electrons are attracted to the positively charged nuclei of both atoms, holding them together.
The Octet Rule and Covalent Bonding
The octet rule, a cornerstone of understanding covalent bonding, states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their outermost electron shell (valence shell). This configuration provides exceptional stability. However, there are exceptions to the octet rule, particularly for elements in the third period and beyond, due to the availability of d orbitals.
Hydrogen, with only one electron, is an exception. It achieves stability with just two electrons in its valence shell – a duet.
Types of Covalent Bonds
Covalent bonds can be categorized based on the number of electron pairs shared:
1. Single Covalent Bonds
A single covalent bond involves the sharing of one electron pair (two electrons) between two atoms. This is represented by a single dash (-) in Lewis structures. A classic example is the bond in a hydrogen molecule (H₂), where each hydrogen atom shares its single electron with the other, completing their respective duets. Similarly, methane (CH₄) showcases four single covalent bonds between the carbon atom and four hydrogen atoms.
2. Double Covalent Bonds
A double covalent bond involves the sharing of two electron pairs (four electrons) between two atoms. This is represented by two dashes (=). A prime example is the bond in oxygen (O₂), where each oxygen atom shares two electrons, resulting in a double bond and fulfilling the octet rule for both atoms. Similarly, carbon dioxide (CO₂) exhibits two double bonds between the carbon atom and each oxygen atom.
3. Triple Covalent Bonds
A triple covalent bond involves the sharing of three electron pairs (six electrons) between two atoms. This is represented by three dashes (≡). Nitrogen gas (N₂) is a quintessential example, where each nitrogen atom shares three electrons, forming a strong triple bond and fulfilling the octet rule for both atoms. The strength of this triple bond contributes to the inertness of nitrogen gas.
Factors Influencing the Number of Shared Electrons
Several factors influence the number of electrons shared in a covalent bond:
-
Valence Electrons: The number of valence electrons an atom possesses determines its bonding capacity. Atoms tend to share electrons to achieve a stable electron configuration, often following the octet rule.
-
Electronegativity: Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. A significant difference in electronegativity between two atoms can lead to a polar covalent bond, where the shared electrons are unevenly distributed. However, even in polar bonds, electron sharing still occurs, though not equally.
-
Atomic Size: Larger atoms generally have weaker covalent bonds due to increased distance between the nuclei.
-
Bond Order: The bond order is the number of bonding electron pairs between two atoms. A higher bond order indicates a stronger and shorter bond, signifying a greater degree of electron sharing.
Beyond the Octet Rule: Exceptions
While the octet rule serves as a useful guideline, there are notable exceptions, primarily involving elements in the third period and beyond (period 3 and below). These elements have access to d orbitals, which can accommodate additional electrons beyond the eight required for the octet rule.
Examples of exceptions include:
-
Phosphorus pentachloride (PCl₅): Phosphorus forms five covalent bonds with five chlorine atoms, exceeding the octet rule.
-
Sulfur hexafluoride (SF₆): Sulfur forms six covalent bonds with six fluorine atoms, significantly exceeding the octet rule.
-
Hypervalent molecules: Molecules with atoms exhibiting more than eight electrons in their valence shell are known as hypervalent molecules.
These exceptions emphasize that the octet rule is a useful simplification but not an absolute law governing all covalent bonding.
Identifying the Number of Shared Electrons: Lewis Structures
Lewis structures, also known as electron dot diagrams, are crucial tools for visualizing the number of shared electrons in covalent bonds. These diagrams represent valence electrons as dots and show how electrons are shared between atoms in a molecule.
To draw a Lewis structure:
-
Calculate the total number of valence electrons: Sum the valence electrons of all atoms in the molecule.
-
Identify the central atom: Usually, the least electronegative atom is the central atom.
-
Arrange atoms and connect them with single bonds: This accounts for two electrons per bond.
-
Distribute remaining electrons to satisfy the octet rule (or duet for hydrogen): Start by placing lone pairs of electrons around the outer atoms, then fill the octet of the central atom.
-
Form multiple bonds (double or triple) if necessary: If the octet rule cannot be satisfied with single bonds, form double or triple bonds by sharing additional electron pairs.
Practical Applications and Examples
Understanding the number of electrons shared in covalent bonds is essential in numerous fields:
-
Organic Chemistry: The vast majority of organic molecules are held together by covalent bonds. Understanding the number of shared electrons helps predict molecular geometry, reactivity, and other properties.
-
Biochemistry: Proteins, carbohydrates, and nucleic acids—the building blocks of life—are complex molecules held together by covalent bonds. Knowing the number of shared electrons is crucial to understanding their structure and function.
-
Materials Science: The properties of many materials, such as polymers and semiconductors, are directly related to the types and strengths of covalent bonds present.
-
Environmental Science: Understanding covalent bonding is crucial for analyzing the behavior of pollutants and understanding chemical reactions in the environment.
Let's illustrate with some examples:
-
Water (H₂O): Oxygen shares two electrons with each hydrogen atom, forming two single covalent bonds. Oxygen has two lone pairs of electrons remaining.
-
Carbon Dioxide (CO₂): Carbon shares four electrons with each oxygen atom, forming two double covalent bonds.
-
Methane (CH₄): Carbon shares two electrons with each hydrogen atom, forming four single covalent bonds.
Conclusion
The number of electrons shared in a covalent bond is a critical aspect of understanding molecular structure and properties. While the octet rule provides a useful framework, exceptions exist, particularly for elements beyond the second period. Lewis structures offer a powerful visual representation of electron sharing, enabling us to predict and analyze the bonding patterns in a diverse range of molecules. The principles discussed here are fundamental to various scientific disciplines and contribute significantly to our comprehension of the material world. A thorough grasp of these concepts is crucial for success in chemistry, biochemistry, and related fields. Further exploration of advanced bonding theories can provide even deeper insights into the complexities of chemical bonding.
Latest Posts
Latest Posts
-
What Is The Value Of 8
Mar 26, 2025
-
How Many Valence Electrons Chlorine Have
Mar 26, 2025
-
What Is Half A Mile In Feet
Mar 26, 2025
-
What Percent Of 12 5 Is 39
Mar 26, 2025
-
Empirical And Molecular Formula Of Ibuprofen
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are Shared In A Covalent Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
