How Many Valence Electrons Chlorine Have
listenit
Mar 26, 2025 · 5 min read
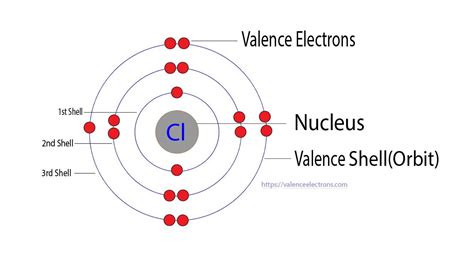
Table of Contents
How Many Valence Electrons Does Chlorine Have? A Deep Dive into Chlorine's Electronic Structure
Chlorine, a crucial element in our everyday lives, plays a vital role in various processes, from water purification to the production of essential chemicals. Understanding its atomic structure, particularly the number of valence electrons it possesses, is key to comprehending its chemical behavior and reactivity. This comprehensive guide will delve deep into the world of chlorine's electronic structure, exploring its valence electrons, their significance, and the implications for chlorine's chemical properties.
Understanding Valence Electrons: The Key to Reactivity
Before diving into chlorine's specific case, let's establish a fundamental understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons involved in chemical bonding and determine an element's reactivity. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to achieve a full outermost shell (typically eight electrons, following the octet rule). This stable configuration is what drives chemical reactions and the formation of molecules.
The Significance of the Octet Rule
The octet rule, while not universally applicable, provides a helpful framework for understanding chemical bonding. It states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight valence electrons, mimicking the stable electron configuration of noble gases. Exceptions exist, especially for elements in the first and second periods (like hydrogen and lithium), but the octet rule remains a valuable guideline for predicting chemical behavior.
Delving into Chlorine's Electronic Structure: Unveiling the Valence Electrons
Chlorine (Cl) has an atomic number of 17, meaning it possesses 17 protons and 17 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁵
This electron configuration reveals the distribution of electrons across different energy levels (shells) and sublevels (orbitals). Let's break it down:
- 1s²: Two electrons in the first energy level (shell)
- 2s²: Two electrons in the second energy level
- 2p⁶: Six electrons in the second energy level (p-orbital)
- 3s²: Two electrons in the third energy level
- 3p⁵: Five electrons in the third energy level (p-orbital)
The outermost shell, the third energy level, contains seven electrons (2 from the 3s and 5 from the 3p). Therefore, chlorine possesses 7 valence electrons.
Visualizing Chlorine's Electron Configuration
Imagine the electrons residing in different shells around the chlorine nucleus. The first shell is closest to the nucleus and can hold a maximum of two electrons. The second shell can hold up to eight electrons (two in the 2s sublevel and six in the 2p sublevel), and the third shell, where the valence electrons reside, can hold up to 18 electrons.
Chlorine's Reactivity: The Role of Valence Electrons
The presence of seven valence electrons makes chlorine highly reactive. To achieve a stable octet, chlorine readily gains one electron, forming a negatively charged chloride ion (Cl⁻). This negatively charged ion is much more stable than the neutral chlorine atom. This tendency to gain an electron is a hallmark of nonmetals, and chlorine exemplifies this characteristic.
Chemical Bonding in Chlorine Compounds
Chlorine's strong tendency to gain an electron drives its participation in various types of chemical bonds:
-
Ionic Bonding: Chlorine readily forms ionic bonds with metals, such as sodium (Na). Sodium, with one valence electron, readily loses this electron to chlorine, forming Na⁺ and Cl⁻ ions, which are held together by electrostatic forces in the ionic compound sodium chloride (NaCl), commonly known as table salt.
-
Covalent Bonding: Chlorine can also form covalent bonds with other nonmetals by sharing electrons. For example, in chlorine gas (Cl₂), two chlorine atoms share one pair of electrons, completing each other's octets. This bond is a strong single covalent bond.
Applications of Chlorine and its Compounds: From Water Purification to Pharmaceuticals
The unique chemical properties of chlorine, stemming from its seven valence electrons, contribute to its wide range of applications. Here are some notable examples:
-
Water Purification: Chlorine's strong oxidizing power makes it an effective disinfectant for water treatment. It kills harmful bacteria and other microorganisms, making water safe for consumption.
-
Industrial Chemicals: Chlorine is a critical component in the production of numerous industrial chemicals, including plastics (PVC), solvents, and refrigerants.
-
Pharmaceuticals: Chlorine is used in the synthesis of numerous pharmaceuticals and medicinal compounds.
-
Bleaching Agents: Chlorine-based compounds are widely used as bleaching agents in the paper, textile, and other industries.
Beyond the Octet Rule: Exceptions and Refinements
While the octet rule serves as a valuable guideline, it’s important to acknowledge its limitations. Some compounds and molecules do not adhere strictly to the octet rule. For instance, certain transition metals can have expanded valence shells accommodating more than eight electrons. Furthermore, molecules like boron trifluoride (BF₃) exhibit electron deficiency, lacking a complete octet. These exceptions underscore the need for a more nuanced understanding of chemical bonding beyond the simple octet rule.
Advanced Concepts: Hybridization and Molecular Geometry
The valence electrons play a crucial role in determining the molecular geometry and hybridization of chlorine-containing compounds. Hybridization, a theoretical concept, describes the mixing of atomic orbitals to form new hybrid orbitals with different energies and shapes. Understanding hybridization is vital for predicting the three-dimensional arrangement of atoms in molecules. For instance, the shape of a molecule like CH₃Cl (chloromethane) can be predicted using the principles of valence shell electron pair repulsion (VSEPR) theory, taking into account the arrangement of valence electrons around the central carbon atom.
Conclusion: The Significance of Valence Electrons in Understanding Chlorine's Chemistry
The number of valence electrons an atom possesses significantly influences its chemical behavior. Chlorine, with its seven valence electrons, readily gains an electron to achieve a stable octet, leading to its high reactivity and numerous applications. Understanding chlorine’s electronic structure is crucial for comprehending its role in various chemical reactions and its widespread use across diverse fields, from water purification to industrial chemical production. This exploration into chlorine's valence electrons highlights the fundamental principles of chemical bonding and the significance of electron configuration in determining an element's properties and reactivity. While the octet rule serves as a valuable starting point, it is important to recognize its limitations and delve into more advanced concepts such as hybridization and VSEPR theory to fully understand the intricacies of chlorine's chemical behavior.
Latest Posts
Latest Posts
-
Standard Enthalpy Of Formation Of Ethanol
Mar 29, 2025
-
Do Parallelograms Have 4 Right Angles
Mar 29, 2025
-
Least Common Multiple Of 10 And 8
Mar 29, 2025
-
Name 3 Ways To Dissolve Something Faster
Mar 29, 2025
-
Charged Language In I Have A Dream
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Chlorine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
