How Many Water Molecules In A Drop
listenit
Mar 25, 2025 · 5 min read
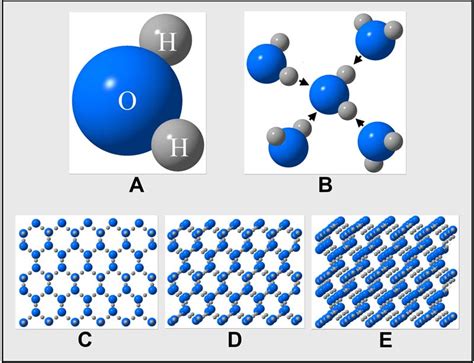
Table of Contents
How Many Water Molecules in a Drop? Diving Deep into a Seemingly Simple Question
The seemingly simple question, "How many water molecules are in a drop of water?" actually opens a fascinating door into the world of chemistry, physics, and even a bit of philosophy. While a precise answer requires specifying the size of the drop, we can delve into the calculations and explore the concepts involved to arrive at a reasonable estimate, and along the way, discover much more about the fundamental building blocks of our world.
Understanding the Building Blocks: Water Molecules (H₂O)
Before we can count water molecules, we need to understand what they are. A water molecule is composed of two hydrogen atoms covalently bonded to a single oxygen atom. This arrangement creates a bent, polar molecule, meaning it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity is crucial for water's unique properties, such as its high boiling point and ability to act as a solvent.
Key Characteristics of a Water Molecule:
- Chemical Formula: H₂O
- Molecular Weight: Approximately 18.015 atomic mass units (amu)
- Polarity: Possesses a slightly positive and negative end due to the electronegativity difference between oxygen and hydrogen.
Defining "A Drop" – The Elusive Variable
The biggest challenge in answering our question lies in defining "a drop." A drop of water isn't a standardized unit of measurement like a liter or a milliliter. Its size varies significantly depending on several factors:
- The method of dispensing: A drop from an eyedropper will differ considerably in size from a drop falling from a leaky faucet or condensation forming on a cold glass.
- Surface tension: Surface tension influences the shape and size of a drop, creating a spherical form that minimizes surface area.
- Viscosity of the liquid: Adding substances to water can change its viscosity, subsequently affecting the size of a drop.
For our calculation, we need to assume a standard drop size. A commonly used approximation is that one drop of water is approximately 0.05 milliliters (mL). This is a reasonable average, but it's crucial to remember that this is just an estimation.
The Calculation: From Milliliters to Molecules
To calculate the number of water molecules in a 0.05 mL drop, we'll need to employ several conversion factors:
-
Milliliters to Grams: First, we need to convert the volume (0.05 mL) into mass (grams). The density of water is approximately 1 gram per milliliter (g/mL). Therefore, 0.05 mL of water weighs approximately 0.05 grams.
-
Grams to Moles: Next, we'll convert the mass of water (0.05 g) into moles using the molar mass of water (18.015 g/mol). The formula is:
Moles = mass (g) / molar mass (g/mol)Moles = 0.05 g / 18.015 g/mol ≈ 0.00277 moles -
Moles to Molecules: Finally, we'll use Avogadro's number (approximately 6.022 x 10²³) to convert moles into the number of molecules. Avogadro's number represents the number of molecules or atoms in one mole of a substance. The formula is:
Number of molecules = moles × Avogadro's numberNumber of molecules ≈ 0.00277 moles × 6.022 x 10²³ molecules/mol ≈ 1.67 x 10²¹ molecules
Therefore, based on our approximation of a 0.05 mL drop, there are approximately 1.67 x 10²¹ water molecules in a single drop.
The Magnitude of the Number – Understanding Scientific Notation
The number 1.67 x 10²¹ is a massive number. Scientific notation is used to express extremely large or small numbers concisely. This notation represents the number as a decimal between 1 and 10, multiplied by a power of 10. In this case, 1.67 x 10²¹ means 1.67 followed by 21 zeros:
1,670,000,000,000,000,000,000
To put this into perspective, this is more than the number of grains of sand on all the beaches of the world! The sheer number of molecules in even a tiny drop of water underlines the scale of the atomic and molecular realm.
Factors Influencing the Accuracy of the Calculation
It's crucial to reiterate that the calculation above provides an estimate. Several factors can influence its accuracy:
- Variations in drop size: As mentioned earlier, the size of a drop is not standardized. Using a larger or smaller drop would yield a different result.
- Temperature: The density of water changes slightly with temperature. Our calculation used the density of water at around 4°C (39°F), which is the temperature at which water has its maximum density.
- Impurities: If the water contains dissolved substances (like salts or minerals), the calculation will be affected. The additional mass of dissolved solids would increase the total mass and thus the number of molecules.
Beyond the Numbers: Exploring the Implications
The sheer number of molecules in even a tiny drop of water highlights the following:
- The scale of the microscopic world: It underscores how incredibly vast the number of atoms and molecules are, even in everyday things.
- The power of chemistry: Understanding the properties of individual water molecules helps us appreciate water’s importance for life on earth.
- Statistical mechanics: Calculations like this are essential in statistical mechanics, a branch of physics that deals with the behaviour of large collections of molecules.
Conclusion: A Journey of Discovery
While a precise answer to "how many water molecules are in a drop?" depends on the exact size of the drop and other factors, our calculations offer a reasonable approximation and a pathway to understanding the concepts involved. This journey has taken us from the molecular structure of water, through the complexities of measurement and scientific notation, to a deeper appreciation of the immense scale of the microscopic world. The seemingly simple question has unveiled a wealth of knowledge, reminding us that even the most basic aspects of our world hold immense complexity and wonder. Remember that this exploration is a starting point; further research into related topics like molarity, solutions, and statistical thermodynamics can enrich your understanding of the molecular world and its quantitative nature.
Latest Posts
Latest Posts
-
What Is The Difference Between Current And Static Electricity
Mar 25, 2025
-
Greatest Common Factor Of 15 And 60
Mar 25, 2025
-
How Many Electrons Are Shared In A Covalent Bond
Mar 25, 2025
-
Groups 3 12 Contain Metals Known As
Mar 25, 2025
-
How Many Atoms Are Required To Form A Molecule
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Water Molecules In A Drop . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
