Is Boiling Water Chemical Or Physical Change
listenit
Mar 17, 2025 · 5 min read
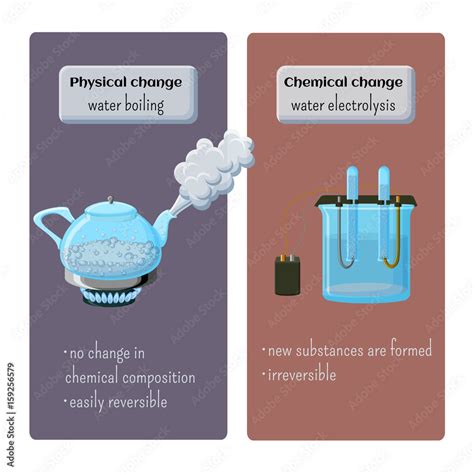
Table of Contents
Is Boiling Water a Chemical or Physical Change? A Deep Dive
The question of whether boiling water represents a chemical or physical change is a classic introductory chemistry conundrum. While seemingly simple, it delves into the fundamental concepts of matter and its transformations. Understanding this distinction is crucial for grasping more complex chemical and physical processes. This comprehensive article will explore the nuances of this question, examining the properties of water, the process of boiling, and the scientific principles involved. We'll also look at some common misconceptions and delve into related examples to solidify your understanding.
Understanding Chemical vs. Physical Changes
Before we dive into the specifics of boiling water, let's establish a clear understanding of the difference between chemical and physical changes.
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state of matter) are modified. These changes are often reversible. Examples include melting ice, cutting paper, dissolving sugar in water, and boiling water.
Chemical Change: A chemical change, also known as a chemical reaction, involves a rearrangement of atoms and molecules to form new substances with different chemical properties. These changes are often irreversible and are accompanied by observable changes, such as a change in color, temperature, or the formation of a gas or precipitate. Examples include burning wood, rusting iron, and baking a cake.
The Process of Boiling Water: A Detailed Look
Boiling water is a phase transition, specifically from the liquid phase to the gaseous phase (water vapor or steam). This transition occurs when the water reaches its boiling point, which is 100°C (212°F) at standard atmospheric pressure. Let's break down what happens at a molecular level:
Molecular Behavior in Boiling Water
-
Liquid Water: In liquid water, water molecules (H₂O) are relatively close together, held by intermolecular forces (hydrogen bonds). These molecules are constantly moving and colliding, but their movement is restricted due to the attractive forces between them.
-
Heating Water: As heat is applied, the kinetic energy of the water molecules increases. This means the molecules move faster and collide more forcefully.
-
Reaching the Boiling Point: At the boiling point, the kinetic energy of the water molecules becomes high enough to overcome the intermolecular forces holding them together in the liquid phase.
-
Vaporization: The water molecules escape the liquid phase and transition into the gaseous phase (steam). This is a phase change, not a chemical change. The molecules themselves remain H₂O.
Observable Changes During Boiling
Several observable changes accompany the boiling of water, further supporting its classification as a physical change:
-
Temperature Remains Constant: While heat is continuously applied, the temperature of the boiling water remains relatively constant at 100°C (at standard atmospheric pressure). This is because the added energy is used to overcome the intermolecular forces, not to increase the kinetic energy of the molecules.
-
Formation of Bubbles: Bubbles of water vapor form within the liquid and rise to the surface, indicating the transition from liquid to gas.
-
Volume Increase: The volume of the water significantly increases as it transitions to steam, showcasing a change in density.
-
Reversible Process: If the steam is cooled, it condenses back into liquid water, demonstrating the reversibility of the process.
Why Boiling Water is a Physical Change
Based on our analysis, the boiling of water definitively qualifies as a physical change. The chemical composition of the water remains unchanged throughout the process. The water molecules are still H₂O, both in liquid and gaseous states. Only the arrangement and energy state of the molecules have changed. The process involves a change of state—a physical transformation—not the creation of new chemical substances.
Addressing Common Misconceptions
Despite the clear scientific explanation, some misconceptions persist regarding boiling water:
Misconception 1: Boiling water produces new gases like oxygen or hydrogen.
Reality: This is incorrect. The steam produced is still composed of water molecules (H₂O). While some impurities might be present in the water, the boiling process itself doesn't break down water molecules into their constituent elements.
Misconception 2: Boiling water alters the chemical properties of water.
Reality: While certain physical properties change (e.g., density, volume), the inherent chemical properties of water—its ability to act as a solvent, its polarity, etc.—remain intact.
Misconception 3: The presence of bubbles indicates a chemical reaction.
Reality: Bubbles are a result of the phase transition; they represent water vapor, not a new chemical product.
Further Examples of Physical Changes: Expanding the Understanding
To solidify the concept of physical change, let's consider other related examples:
-
Melting Ice: Ice (solid water) melts into liquid water. The chemical composition remains H₂O.
-
Freezing Water: Liquid water freezes into ice. Again, the chemical structure remains the same.
-
Sublimation of Dry Ice: Solid carbon dioxide (dry ice) changes directly into gaseous carbon dioxide without passing through the liquid phase. The chemical identity remains CO₂.
-
Evaporation of Alcohol: Liquid alcohol evaporates into gaseous alcohol. The alcohol molecules remain intact.
Conclusion: The Boiling Point and Beyond
Boiling water provides a clear and straightforward example of a physical change. The process involves a change in state—from liquid to gas—without altering the chemical composition of the substance. Understanding this fundamental distinction is vital in many scientific disciplines, from chemistry and physics to engineering and environmental science. By comprehending the molecular behavior during boiling and comparing it with chemical changes, we gain a deeper appreciation for the fundamental principles governing matter and its transformations. This knowledge helps us interpret various phenomena in the natural world and lays the foundation for further exploration in the realms of chemistry and physics. The seemingly simple act of boiling water thus serves as a potent illustration of the power of scientific observation and the importance of distinguishing between physical and chemical processes.
Latest Posts
Latest Posts
-
Lewis Acid And Base Vs Bronsted
Mar 17, 2025
-
How Are A Mole And A Dozen Similar
Mar 17, 2025
-
What Is The Percentage Of 0 2
Mar 17, 2025
-
Integral 1 X 1 X 2
Mar 17, 2025
-
Sodium Hydroxide And Sulfuric Acid Balanced Equation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
