Sodium Hydroxide And Sulfuric Acid Balanced Equation
listenit
Mar 17, 2025 · 5 min read
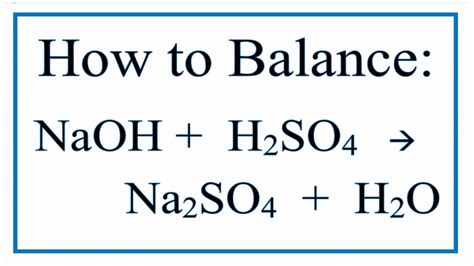
Table of Contents
Sodium Hydroxide and Sulfuric Acid: A Balanced Equation and Beyond
The reaction between sodium hydroxide (NaOH), a strong base, and sulfuric acid (H₂SO₄), a strong acid, is a classic example of a neutralization reaction. Understanding this reaction, its balanced equation, and the implications of its products is crucial in various fields, from chemistry education to industrial processes. This comprehensive article will delve into the details of this reaction, exploring its balanced equation, stoichiometry, applications, safety precautions, and related concepts.
Understanding the Reaction: Neutralization
Neutralization reactions are characterized by the reaction between an acid and a base, resulting in the formation of salt and water. In this specific case, the strong acid, sulfuric acid, donates protons (H⁺ ions) to the strong base, sodium hydroxide, which accepts these protons. This proton transfer leads to the formation of water and a salt, sodium sulfate.
The Reactants: Sodium Hydroxide and Sulfuric Acid
-
Sodium Hydroxide (NaOH): Also known as caustic soda or lye, NaOH is a highly alkaline substance readily soluble in water. It's widely used in various industries, including soap making, paper production, and drain cleaning. Its strong basicity stems from its ability to readily dissociate into Na⁺ and OH⁻ ions in aqueous solutions.
-
Sulfuric Acid (H₂SO₄): A highly corrosive strong mineral acid, sulfuric acid is a crucial industrial chemical used extensively in fertilizer production, petroleum refining, and metal processing. Its strong acidity is due to its ability to donate two protons (H⁺ ions) per molecule.
Balancing the Chemical Equation
The balanced chemical equation represents the quantitative relationship between reactants and products in a chemical reaction. It ensures that the number of atoms of each element is equal on both sides of the equation, reflecting the law of conservation of mass. The unbalanced equation for the reaction between sodium hydroxide and sulfuric acid is:
NaOH + H₂SO₄ → Na₂SO₄ + H₂O
This equation is unbalanced because the number of sodium (Na), hydrogen (H), and oxygen (O) atoms are not equal on both sides. To balance it, we need to adjust the stoichiometric coefficients:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
This balanced equation shows that two moles of sodium hydroxide react with one mole of sulfuric acid to produce one mole of sodium sulfate and two moles of water.
Stoichiometry and Calculations
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. Using the balanced equation, we can perform various stoichiometric calculations, such as determining the amount of product formed from a given amount of reactant or determining the limiting reactant in a reaction.
For example, if we have 10 moles of NaOH, we can calculate the number of moles of H₂SO₄ needed for complete reaction:
From the balanced equation: 2 moles NaOH react with 1 mole H₂SO₄.
Therefore, 10 moles NaOH will react with (10 moles NaOH) * (1 mole H₂SO₄ / 2 moles NaOH) = 5 moles H₂SO₄.
Similarly, we can calculate the amount of Na₂SO₄ and H₂O produced.
Applications of the Reaction
The neutralization reaction between sodium hydroxide and sulfuric acid has numerous applications across various industries:
-
Wastewater Treatment: This reaction is used to neutralize acidic wastewater from industrial processes, making it less harmful before disposal.
-
Chemical Synthesis: The reaction is a crucial step in various chemical syntheses, where precise control over pH is required.
-
pH Control: In various industrial processes, maintaining a specific pH is crucial. The controlled addition of either NaOH or H₂SO₄ can be used to adjust the pH to the desired level.
-
Production of Sodium Sulfate: Sodium sulfate, a product of this reaction, has various applications as a desiccant, in detergents, and in the pulp and paper industry.
Safety Precautions
Both sodium hydroxide and sulfuric acid are highly corrosive and hazardous chemicals. Handling them requires strict adherence to safety protocols:
-
Protective Gear: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, lab coats, and possibly respirators, depending on the concentration and scale of the reaction.
-
Ventilation: The reaction should be carried out in a well-ventilated area to prevent inhalation of fumes.
-
Slow Addition: Always add the acid to the base slowly and carefully, stirring continuously to prevent splashing and localized heating. Adding base to acid can be extremely dangerous due to the potential for violent exothermic reactions.
-
Emergency Procedures: Have appropriate emergency procedures and equipment readily available in case of spills or accidents.
Related Concepts and Further Exploration
Understanding the reaction between sodium hydroxide and sulfuric acid provides a strong foundation for exploring more advanced concepts in chemistry:
-
Titration: This reaction is frequently used in titrations to determine the concentration of an unknown acid or base solution.
-
Acid-Base Equilibria: The reaction helps illustrate the principles of acid-base equilibria and the concept of pH.
-
Thermochemistry: The reaction is exothermic, meaning it releases heat. Studying the heat released can provide insights into the thermodynamics of the reaction.
-
Electrochemistry: The reaction can be used to illustrate the principles of electrochemical cells and the generation of electricity from chemical reactions.
Conclusion
The neutralization reaction between sodium hydroxide and sulfuric acid is a fundamental chemical process with wide-ranging applications. Understanding the balanced equation, stoichiometry, safety precautions, and related concepts is essential for anyone working with these chemicals. This comprehensive exploration provides a strong foundation for further learning and practical applications in chemistry and related fields. Remember, safety should always be the top priority when working with strong acids and bases. Always consult relevant safety data sheets (SDS) and follow established laboratory safety protocols.
Latest Posts
Latest Posts
-
What Is The Square Root Of 23
Mar 17, 2025
-
Is Naoh A Acid Or Base
Mar 17, 2025
-
How Many Ounces Is A 2 L
Mar 17, 2025
-
How Much Is 120 Inches In Feet
Mar 17, 2025
-
2x 2 7x 9 0 Quadratic Formula
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Sodium Hydroxide And Sulfuric Acid Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
