Is Baking Soda Ionic Or Covalent
listenit
Mar 24, 2025 · 6 min read
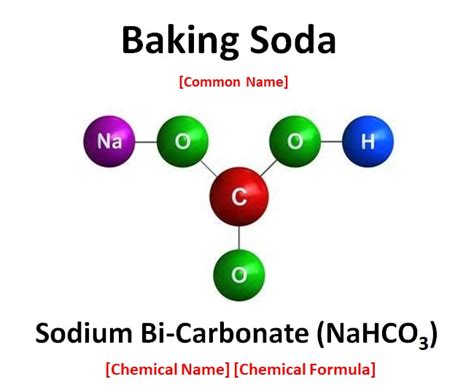
Table of Contents
Is Baking Soda Ionic or Covalent? Delving into the Chemistry of Sodium Bicarbonate
Baking soda, a ubiquitous kitchen staple, is more than just a leavening agent; it's a fascinating chemical compound with a unique structure that determines its properties. The question of whether baking soda (sodium bicarbonate, NaHCO₃) is ionic or covalent is a common one, and the answer isn't as simple as a straightforward "yes" or "no." It's a nuanced situation involving a blend of both ionic and covalent bonding, making it a great example of the complexities of chemical bonding. This comprehensive guide will explore the chemical structure of baking soda, examine the different types of bonds present, and ultimately answer the question of its bonding nature.
Understanding Ionic and Covalent Bonds
Before we delve into the specifics of baking soda, let's refresh our understanding of ionic and covalent bonds.
Ionic Bonds: The Electrostatic Attraction
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This happens when one atom, typically a metal, loses one or more electrons to become a positively charged ion (cation), and another atom, usually a non-metal, gains those electrons to become a negatively charged ion (anion). The strong attraction between these oppositely charged ions forms the ionic bond. Think of magnets: opposite poles attract. This results in a crystal lattice structure, a rigid three-dimensional arrangement of ions. Examples include sodium chloride (NaCl, table salt) and magnesium oxide (MgO).
Covalent Bonds: Sharing is Caring
Covalent bonds, on the other hand, involve the sharing of electrons between two atoms. This usually happens between non-metal atoms. Instead of one atom completely losing or gaining electrons, atoms share electrons to achieve a stable electron configuration, often resembling that of a noble gas. This sharing creates a strong bond between the atoms. Examples include water (H₂O) and methane (CH₄).
Polar Covalent Bonds: A Spectrum of Sharing
Within covalent bonds, there's a spectrum of sharing. In a nonpolar covalent bond, electrons are shared equally between atoms of similar electronegativity (the tendency of an atom to attract electrons). In a polar covalent bond, electrons are shared unequally, resulting in a slightly positive end (δ+) and a slightly negative end (δ−). This occurs when atoms have different electronegativities. The greater the difference in electronegativity, the more polar the bond.
The Chemical Structure of Sodium Bicarbonate (NaHCO₃)
Baking soda, or sodium bicarbonate, has a more complex structure than simple ionic or covalent compounds. Its formula, NaHCO₃, reveals that it contains:
- One sodium ion (Na⁺): A metal cation that readily loses one electron.
- One bicarbonate ion (HCO₃⁻): A polyatomic anion containing multiple atoms bonded together.
The key to understanding the bonding in NaHCO₃ lies within the bicarbonate ion.
The Bicarbonate Ion (HCO₃⁻): A Covalent Backbone with Ionic Interactions
The bicarbonate ion, HCO₃⁻, is where the covalent and ionic bonding interplay is most apparent. Within the bicarbonate ion:
-
Covalent bonds: Carbon (C) forms covalent bonds with three oxygen atoms (O) and one hydrogen atom (H). These bonds are polar covalent bonds due to the difference in electronegativity between carbon and oxygen. The oxygen atoms are more electronegative, leading to a partial negative charge (δ−) on the oxygen atoms and a partial positive charge (δ+) on the carbon and hydrogen atoms. The structure involves a central carbon atom double-bonded to one oxygen atom and single-bonded to two other oxygen atoms. One of the single-bonded oxygen atoms is also bonded to a hydrogen atom, forming a hydroxyl group (-OH).
-
Resonance Structures: The actual structure of the bicarbonate ion is best represented by resonance structures. This means the electrons are delocalized across multiple bonds, resulting in an average bond order that is between a single and a double bond. This delocalization stabilizes the molecule and makes it more resilient.
-
Charge Distribution: The overall negative charge on the bicarbonate ion (HCO₃⁻) is delocalized across the oxygen atoms.
The Ionic Interaction in Baking Soda
The sodium ion (Na⁺) and the bicarbonate ion (HCO₃⁻) are held together by an ionic bond. The positively charged sodium ion is electrostatically attracted to the negatively charged bicarbonate ion. This strong electrostatic attraction is what gives baking soda its crystalline structure.
Is Baking Soda Ionic or Covalent? The Verdict
Therefore, the answer to the question "Is baking soda ionic or covalent?" is both. Baking soda exhibits both ionic and covalent bonding. The bicarbonate ion itself is held together by covalent bonds, while the sodium ion and the bicarbonate ion are held together by an ionic bond. This is a common feature in many polyatomic ionic compounds. The predominant bonding is ionic, as this is the primary force holding the sodium and bicarbonate ions together in the crystal lattice. However, the internal structure of the bicarbonate ion involves covalent bonds which are essential for the overall behavior of the compound.
The Implications of Baking Soda's Bonding
The dual nature of baking soda's bonding has significant implications for its properties and uses:
-
Solubility: Baking soda is soluble in water because the ionic bonds between Na⁺ and HCO₃⁻ are broken by the polar water molecules. The water molecules surround and stabilize the ions, allowing them to separate and dissolve.
-
Reactivity: The bicarbonate ion's structure allows it to act as a weak base, reacting with acids to produce carbon dioxide gas. This is the basis of its leavening action in baking. The covalent bonds within the bicarbonate ion are rearranged during this reaction.
-
Crystal Structure: The ionic interactions between Na⁺ and HCO₃⁻ create a specific crystal lattice structure, dictating the physical properties of baking soda like its powdery nature and density.
Baking Soda's Versatile Applications
Understanding the chemical structure of baking soda helps to understand its wide array of applications:
- Baking: As mentioned before, its reaction with acids produces carbon dioxide, making baked goods rise.
- Cleaning: Its mildly basic nature helps neutralize acids and break down grease and grime.
- Deodorizing: It absorbs odors by neutralizing acidic compounds.
- Health and Beauty: It is used in some toothpastes due to its mild abrasiveness and ability to neutralize acids in the mouth.
Conclusion
Baking soda's chemical composition is a testament to the complexity and diversity of chemical bonding. It's not a simple case of solely ionic or solely covalent bonding; instead, it’s a fascinating example of how both ionic and covalent interactions work together to determine a compound's properties. Its dual nature is responsible for its wide range of applications, making it a truly remarkable and versatile substance. The next time you use baking soda in your kitchen or for cleaning, remember the intricate dance of ions and molecules that makes it so effective. Understanding this fundamental chemistry unlocks a deeper appreciation for the seemingly simple everyday substance that is baking soda.
Latest Posts
Latest Posts
-
What Is 1 3 Plus 1 4
Mar 25, 2025
-
The Study Of The Cells Is Called
Mar 25, 2025
-
Can Mixtures Be Separated By Physical Means
Mar 25, 2025
-
Which State Of Matter Has The Highest Kinetic Energy
Mar 25, 2025
-
What Is The Opposite Of Sublimation
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda Ionic Or Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
