Is Alcohol A Acid And A Base Bronsted
listenit
Mar 23, 2025 · 6 min read
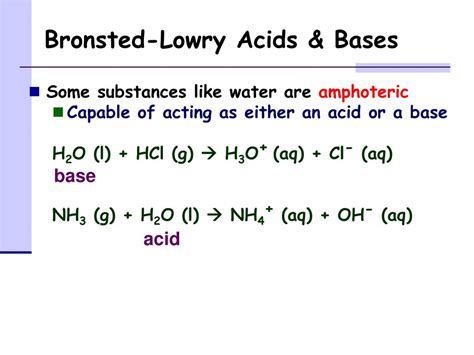
Table of Contents
Is Alcohol an Acid and a Base (Brønsted-Lowry)? A Deep Dive into Acid-Base Chemistry
The question of whether alcohol is an acid or a base, particularly within the framework of the Brønsted-Lowry theory, requires a nuanced understanding of acid-base chemistry. While not as strong as many common acids or bases, alcohols exhibit amphoteric behavior, meaning they can act as both an acid and a base, depending on the reaction conditions. This article will explore the amphoteric nature of alcohols, examining their acidic and basic properties in detail, and providing examples to illustrate their behavior in different chemical contexts.
Understanding the Brønsted-Lowry Definition
Before delving into the specifics of alcohols, it's crucial to revisit the Brønsted-Lowry definition of acids and bases. This theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry, defines an acid as a proton (H⁺) donor and a base as a proton (H⁺) acceptor. This definition broadens the scope of acid-base reactions beyond the limitations of the Arrhenius theory, which only considers reactions involving H⁺ and OH⁻ ions in aqueous solutions.
The Brønsted-Lowry theory is fundamental to understanding the acid-base behavior of alcohols because it explains how they can participate in both proton donation and acceptance.
Alcohols as Acids: Proton Donation
Alcohols, characterized by the hydroxyl (-OH) functional group, can act as weak acids. The hydroxyl hydrogen is relatively acidic due to the electronegativity of the oxygen atom. The oxygen atom pulls electron density away from the O-H bond, making the hydrogen more readily donated as a proton.
This proton donation can occur in the presence of a strong enough base. The reaction involves the alcohol donating a proton to the base, forming an alkoxide ion (RO⁻) and the conjugate acid of the base.
Example: The reaction between ethanol (CH₃CH₂OH) and sodium hydride (NaH):
CH₃CH₂OH + NaH → CH₃CH₂O⁻Na⁺ + H₂
In this reaction, ethanol acts as an acid, donating a proton to the hydride ion (H⁻), a strong base. The resulting ethoxide ion (CH₃CH₂O⁻) is the conjugate base of ethanol, and hydrogen gas (H₂) is formed. Sodium hydride is extremely reactive and used sparingly; other strong bases can also facilitate this reaction.
Factors Affecting Acidity of Alcohols:
Several factors influence the acidity of alcohols:
-
Inductive Effects: Electron-withdrawing groups attached to the carbon atom bonded to the hydroxyl group increase the acidity of the alcohol. These groups stabilize the negative charge on the alkoxide ion, making proton donation more favorable.
-
Steric Effects: Bulky groups around the hydroxyl group can hinder the approach of a base, thus reducing the acidity.
-
Solvent Effects: The solvent can influence the stability of the alkoxide ion, affecting the overall acidity. Protic solvents, which can form hydrogen bonds, tend to stabilize alkoxide ions, whereas aprotic solvents do not.
Alcohols as Bases: Proton Acceptance
Alcohols can also act as weak bases, accepting a proton from a strong enough acid. The oxygen atom in the hydroxyl group possesses lone pairs of electrons that can accept a proton.
Example: The reaction between ethanol (CH₃CH₂OH) and a strong acid like sulfuric acid (H₂SO₄):
CH₃CH₂OH + H₂SO₄ → CH₃CH₂OH₂⁺ + HSO₄⁻
In this reaction, ethanol acts as a base, accepting a proton from sulfuric acid. The resulting protonated ethanol (CH₃CH₂OH₂⁺) is the conjugate acid of ethanol, and the bisulfate ion (HSO₄⁻) is the conjugate base of sulfuric acid.
Factors Affecting Basicity of Alcohols:
The basicity of alcohols is primarily determined by:
-
Availability of Lone Pairs: The lone pairs of electrons on the oxygen atom are crucial for proton acceptance. Anything reducing the availability of these lone pairs will decrease the basicity.
-
Steric Effects: Similar to acidity, bulky groups can hinder the approach of a proton, reducing basicity.
-
Solvent Effects: The solvent can affect the solvation of both the alcohol and the protonated alcohol, influencing the equilibrium of the reaction.
Comparing the Acidic and Basic Strengths of Alcohols
It's important to note that alcohols are considerably weaker acids and weaker bases than many other compounds. Their acidic and basic properties are often only apparent under specific reaction conditions, usually involving exceptionally strong acids or bases. They are not strong enough to significantly alter the pH of most solutions.
Their relative weakness compared to other acids and bases means that their roles as acids or bases are typically secondary or contextual within a reaction. This doesn't diminish their amphoteric nature, however; it merely highlights the need for specific reaction conditions to observe these properties.
Alcohols in Different Chemical Contexts: Demonstrating Amphoteric Behavior
Let's explore some specific chemical scenarios showcasing the amphoteric nature of alcohols:
-
Reaction with Alkali Metals: Reaction with alkali metals (like sodium or potassium) demonstrates the acidic nature of alcohols. The alcohol donates a proton to the metal, forming an alkoxide salt and releasing hydrogen gas.
-
Reaction with Strong Acids: Reaction with strong acids like sulfuric acid or hydrochloric acid highlights the basic character of alcohols. The alcohol accepts a proton from the strong acid, resulting in a protonated alcohol species.
-
Esterification: Esterification reactions involve the reaction of an alcohol with a carboxylic acid in the presence of an acid catalyst. In this reaction, the alcohol acts as a nucleophile (a base) attacking the carbonyl carbon of the carboxylic acid. While the overall reaction doesn't explicitly show the alcohol acting as a Brønsted base (by accepting a proton), the initial nucleophilic attack relies on the availability of the electron pair on the oxygen.
-
Dehydration: The dehydration of alcohols (the removal of water) to form alkenes typically requires a strong acid catalyst. This reaction highlights the alcohol's ability to act both as an acid (losing a proton) and a base (accepting a proton from the acid catalyst). While the proton loss is the direct step that causes the elimination to proceed, the acceptance of a proton on the oxygen facilitates the reaction in several ways.
Applications of Alcohols' Acid-Base Properties
The amphoteric nature of alcohols, while subtle, finds important applications in various chemical processes:
-
Solvent Selection: Alcohols are frequently used as solvents in chemical reactions due to their ability to act as both acids and bases. This allows them to interact with a wide range of reactants.
-
Catalyst Participation: Their ability to donate or accept protons can influence reaction mechanisms in catalytic processes.
-
Synthesis of Organic Compounds: Their amphoteric behavior is pivotal in many organic synthesis pathways, often playing a crucial role in reaction intermediates and selectivity.
Conclusion: The Amphoteric Nature of Alcohols
In conclusion, alcohols exhibit amphoteric behavior, acting as both weak acids and weak bases according to the Brønsted-Lowry definition. Their acidic character stems from the relatively acidic hydroxyl hydrogen, readily donated in the presence of a strong base, forming an alkoxide ion. Their basic character is manifested by the lone pairs of electrons on the oxygen atom, capable of accepting a proton from a strong acid, leading to the formation of a protonated alcohol. Understanding their amphoteric behavior is vital in predicting and controlling their reactivity in diverse chemical contexts, and highlights their versatility in various chemical applications. While their acidity and basicity are weak compared to many other species, their ability to participate in both types of reactions adds to their importance as solvents and reagents in many industrial and laboratory settings. Their behavior in these reactions depends heavily on the reactivity of the other species involved. It is the interplay of these factors that truly demonstrates the amphoteric nature of alcohols within the framework of the Brønsted-Lowry acid-base theory.
Latest Posts
Latest Posts
-
Molarity Of Acetic Acid In Vinegar
Mar 25, 2025
-
How Many Minutes Are In 1 Week
Mar 25, 2025
-
How Many 3rds In A Cup
Mar 25, 2025
-
Can Travel Through A Empty Space
Mar 25, 2025
-
How To Graph A Rose Curve
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Alcohol A Acid And A Base Bronsted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
