In A Double Covalent Bond A Carbon Atom Shares
listenit
Mar 26, 2025 · 6 min read
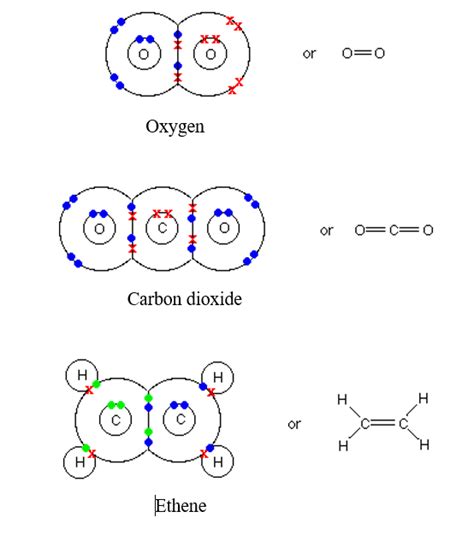
Table of Contents
In a Double Covalent Bond, a Carbon Atom Shares…
Carbon, the backbone of organic life, possesses a unique ability to form stable and diverse molecules through covalent bonding. Understanding how carbon atoms share electrons, particularly in double covalent bonds, is fundamental to comprehending the vast complexity of organic chemistry. This article delves deep into the intricacies of carbon's double bonding behavior, exploring its implications for molecular structure, reactivity, and the properties of countless organic compounds.
The Nature of Covalent Bonding
Before we delve into the specifics of double covalent bonds involving carbon, let's refresh our understanding of covalent bonding itself. Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration, usually a full outer electron shell (octet rule). This sharing occurs between atoms with similar electronegativities, meaning neither atom has a significantly stronger pull on the shared electrons.
Single Covalent Bonds
In a single covalent bond, two atoms share one pair of electrons. A simple example is methane (CH₄), where each hydrogen atom shares one electron with the carbon atom, resulting in four single C-H bonds. This arrangement fulfills the octet rule for carbon (eight valence electrons) and the duet rule for each hydrogen atom (two valence electrons).
Double Covalent Bonds
A double covalent bond, however, involves the sharing of two pairs of electrons between two atoms. This results in a stronger and shorter bond compared to a single bond. In the context of carbon, a double bond significantly impacts the geometry and reactivity of the molecule. Carbon's ability to form double bonds is a key factor in the diversity of organic molecules.
Carbon's Role in Double Covalent Bonds
Carbon's unique position in the periodic table, with four valence electrons, allows it to readily form four covalent bonds. This versatility enables carbon to participate in a wide array of single, double, and even triple bonds. When carbon forms a double bond, it shares two pairs of its valence electrons with another atom, typically carbon, oxygen, nitrogen, or sulfur.
Examples of Carbon's Double Bonds
-
Ethene (C₂H₄): This simple alkene contains a carbon-carbon double bond. Each carbon atom shares two electrons with the other carbon atom and one electron each with two hydrogen atoms. The double bond creates a planar geometry around the carbon atoms.
-
Carbon Dioxide (CO₂): Each carbon atom forms two double bonds, one with each oxygen atom. This linear molecule is essential in biological processes and the Earth's atmosphere.
-
Formaldehyde (H₂CO): The carbon atom forms a double bond with an oxygen atom and two single bonds with hydrogen atoms. Formaldehyde is a crucial building block in the synthesis of various polymers.
-
Acetic Acid (CH₃COOH): This important organic acid features a carbon-oxygen double bond (carbonyl group) and is responsible for the characteristic sour taste of vinegar.
Consequences of Double Bonds on Molecular Structure
The presence of double bonds significantly influences the molecular structure and geometry:
-
Planar Geometry: Double bonds tend to restrict rotation around the bond axis, resulting in a planar or nearly planar arrangement of atoms around the double-bonded atoms. This is crucial for determining molecular shape and reactivity.
-
Bond Length and Strength: Double bonds are shorter and stronger than single bonds due to the increased electron density between the two atoms. This enhanced bond strength impacts the molecule's stability and reactivity.
-
Resonance Structures: In some molecules with multiple double bonds or alternating single and double bonds (conjugated systems), electron delocalization occurs, leading to resonance structures. This phenomenon stabilizes the molecule and affects its chemical properties. Benzene (C₆H₆) is a classic example of a molecule exhibiting resonance.
Double Bonds and Reactivity
The presence of double bonds profoundly influences a molecule's reactivity. The increased electron density in the double bond makes it a site of high electron density, making it susceptible to electrophilic attack.
Electrophilic Addition
A common reaction of molecules with carbon-carbon double bonds is electrophilic addition. Electrophiles, electron-deficient species, are attracted to the electron-rich double bond and react to form new single bonds. This reaction is crucial in many industrial processes and biological pathways.
Oxidation and Reduction
Double bonds can also undergo oxidation and reduction reactions. Oxidation involves the addition of oxygen atoms or the removal of hydrogen atoms, while reduction involves the addition of hydrogen atoms or the removal of oxygen atoms. These reactions are important in metabolic pathways and organic synthesis.
Polymerization
Double bonds play a crucial role in polymerization, a process that joins many small molecules (monomers) to form long chains (polymers). The double bond breaks, allowing monomers to link together, forming strong and stable polymer chains. Polyethylene, a common plastic, is a prime example of a polymer formed through the polymerization of ethene.
Specific Examples in Depth
Let's examine some specific examples to illustrate the influence of carbon's double bonds:
1. Ethene (Ethylene): A Simple Alkene
Ethene (C₂H₄) is the simplest alkene, featuring a carbon-carbon double bond. This double bond is responsible for its planar structure and its reactivity towards electrophilic addition. Ethene's reactivity allows it to participate in polymerization, producing polyethylene.
2. Carbon Dioxide (CO₂): A Linear Molecule
Carbon dioxide (CO₂) possesses two carbon-oxygen double bonds. This linear structure arises from the strong double bonds, impacting its interactions with other molecules and its role in climate change. The molecule's stability is a consequence of the strong double bonds.
3. Benzene (C₆H₆): Aromatic Stability through Resonance
Benzene (C₆H₆) is an aromatic hydrocarbon featuring a ring of six carbon atoms with alternating single and double bonds. However, due to resonance, the electrons are delocalized across the entire ring, leading to exceptional stability and unique chemical properties. This stability makes benzene a crucial starting material in many organic syntheses.
Double Bonds and Spectroscopy
Various spectroscopic techniques are used to detect and characterize double bonds:
-
Infrared (IR) Spectroscopy: The presence of a carbon-carbon double bond results in a characteristic absorption band in the IR spectrum at around 1600-1680 cm⁻¹.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹³C NMR spectroscopy can distinguish between carbon atoms in single and double bonds based on their chemical shifts.
-
Ultraviolet-Visible (UV-Vis) Spectroscopy: Conjugated double bonds absorb UV-Vis light, leading to characteristic absorption peaks that can be used to identify and quantify these systems.
Conclusion
In conclusion, the ability of carbon atoms to share electrons in double covalent bonds is a cornerstone of organic chemistry. The implications of these bonds on molecular structure, geometry, reactivity, and properties are far-reaching. Understanding the intricate interplay of double bonds is essential for comprehending the vast diversity and complexity of the organic world. From the simplest alkenes to complex biological molecules and synthetic polymers, the influence of carbon's double bond is undeniable, making it a fundamental concept for anyone studying chemistry or related fields. The strength, length, and reactivity associated with these bonds shape the world around us, driving advancements in countless areas from medicine to materials science. Further research into the intricacies of carbon double bonds promises to unlock new possibilities and applications.
Latest Posts
Latest Posts
-
What Is Square Root Of 40
Mar 29, 2025
-
How To Factor 1 X 3
Mar 29, 2025
-
How To Find A Boiling Point
Mar 29, 2025
-
What Is 85 In Fraction Form
Mar 29, 2025
-
The Coefficients In A Chemical Equation Represent The
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about In A Double Covalent Bond A Carbon Atom Shares . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
