In A Chemical Reaction Atoms Are
listenit
Mar 30, 2025 · 6 min read
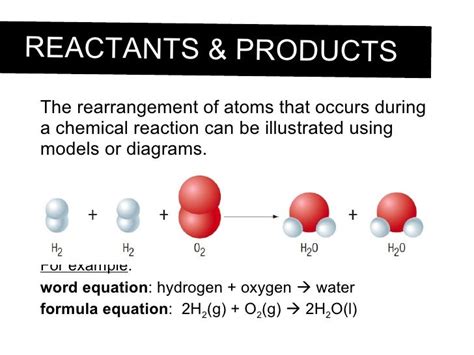
Table of Contents
In a Chemical Reaction, Atoms Are… Rearranged, Not Created or Destroyed
The fundamental principle governing all chemical reactions is the conservation of mass. This seemingly simple statement holds profound implications for our understanding of how matter interacts at the atomic level. In essence, it declares that in a chemical reaction, atoms are neither created nor destroyed; they are simply rearranged. This rearrangement leads to the formation of new substances with different properties, but the total number and type of atoms remain constant. Let's delve deeper into this crucial concept and explore its ramifications.
Understanding Atoms: The Building Blocks of Matter
Before exploring how atoms behave in chemical reactions, it's crucial to understand their nature. Atoms are the fundamental units of matter, incredibly tiny particles composed of a dense nucleus containing protons and neutrons, orbited by electrons. The number of protons defines an element, determining its unique chemical properties. For instance, an atom with one proton is hydrogen, while an atom with six protons is carbon. Isotopes, variations of the same element with different numbers of neutrons, also exist.
The Role of Electrons in Chemical Reactions
Electrons, the negatively charged particles surrounding the nucleus, play the starring role in chemical reactions. Electrons are arranged in shells or energy levels, and the outermost shell, known as the valence shell, contains valence electrons. These valence electrons are responsible for the chemical behavior of an atom, as they are the ones that interact with other atoms during chemical reactions. Atoms strive to achieve a stable electron configuration, often by filling their valence shells completely. This drive towards stability is the driving force behind many chemical reactions.
The Dance of Atoms: How Rearrangement Occurs
Chemical reactions involve the breaking and forming of chemical bonds. A chemical bond is the force of attraction that holds atoms together. The type of bond formed depends on the interaction between the valence electrons of participating atoms. Common types of bonds include:
1. Ionic Bonds: An Electron Transfer
Ionic bonds are formed through the transfer of electrons from one atom to another. This transfer results in the formation of ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. A classic example is the formation of sodium chloride (NaCl), or common table salt, where sodium (Na) loses an electron to chlorine (Cl), forming Na+ and Cl- ions that attract each other.
2. Covalent Bonds: Electron Sharing
Covalent bonds are formed through the sharing of electrons between atoms. This sharing allows both atoms to achieve a more stable electron configuration. Covalent bonds are prevalent in many organic molecules, forming the backbone of life. For example, in a water molecule (H₂O), each hydrogen atom shares an electron with the oxygen atom, resulting in a stable structure.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals and are characterized by a "sea" of delocalized electrons that are shared amongst a lattice of positively charged metal ions. This arrangement allows for the unique properties of metals such as conductivity and malleability.
Evidence for the Conservation of Mass
The principle that atoms are merely rearranged in a chemical reaction is supported by numerous experimental observations and scientific laws:
-
The Law of Conservation of Mass: This fundamental law, first proposed by Antoine Lavoisier, states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products. This directly supports the idea that atoms are neither created nor destroyed, only rearranged. Any apparent change in mass is typically due to the loss or gain of gases involved in the reaction.
-
Stoichiometry: Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It's based on the principle of conservation of mass and allows us to predict the amounts of products formed based on the amounts of reactants used. This would not be possible if atoms were created or destroyed.
-
Nuclear Chemistry vs. Chemical Reactions: It is crucial to distinguish between chemical reactions and nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, resulting in the formation of new elements. In contrast, chemical reactions only involve the rearrangement of electrons and the formation and breaking of chemical bonds, leaving the atomic nuclei unchanged.
Illustrative Examples of Atomic Rearrangement
Let's examine a few examples to further illustrate how atoms are rearranged in chemical reactions:
1. Combustion of Methane (CH₄):
The combustion of methane, a common component of natural gas, involves the reaction of methane with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O).
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction, the carbon and hydrogen atoms from methane combine with oxygen atoms to form new molecules of carbon dioxide and water. Notice that the number of each type of atom remains the same on both sides of the equation.
2. Formation of Water (H₂O):
The formation of water from hydrogen and oxygen is another classic example.
2H₂ + O₂ → 2H₂O
Two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. Again, the atoms are simply rearranged, with the same number of hydrogen and oxygen atoms on both sides of the equation.
3. Neutralization Reaction:
The reaction between an acid and a base, often called a neutralization reaction, involves the exchange of ions. For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl + NaOH → NaCl + H₂O
The hydrogen ion (H+) from the acid combines with the hydroxide ion (OH-) from the base to form water, while the sodium (Na+) and chloride (Cl-) ions combine to form sodium chloride.
Implications of Atomic Rearrangement
The understanding that atoms are rearranged, not created or destroyed, in chemical reactions has profound implications:
-
Environmental Chemistry: Understanding how atoms rearrange during environmental processes, such as combustion and pollution formation, allows us to develop strategies for mitigating environmental damage.
-
Industrial Chemistry: The design and optimization of industrial processes rely heavily on understanding stoichiometry and how atoms rearrange to form desired products.
-
Biochemical Processes: All biological processes are based on chemical reactions, where atoms are rearranged to synthesize biomolecules, break down nutrients, and carry out life's essential functions.
-
Material Science: The development of new materials with specific properties hinges on controlling how atoms arrange themselves in different structures.
Conclusion: The Unwavering Principle
The principle that in a chemical reaction, atoms are rearranged, not created or destroyed, is a cornerstone of chemistry. This understanding underpins countless scientific advancements and technological innovations. From understanding environmental processes to designing new materials, the rearrangement of atoms governs the world around us at its most fundamental level. Appreciating this principle allows for a deeper comprehension of the intricate processes that shape our world. It's a testament to the elegant simplicity and profound power of fundamental scientific laws.
Latest Posts
Latest Posts
-
What Are The Factors For 23
Apr 01, 2025
-
How Many Membranes Surround A Chloroplast
Apr 01, 2025
-
Find Unit Vector Orthogonal To Two Vectors
Apr 01, 2025
-
A Substance That Is Dissolved In A Solution
Apr 01, 2025
-
How Are Electromagnetic Waves Different From Other Waves
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about In A Chemical Reaction Atoms Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
