A Substance That Is Dissolved In A Solution
listenit
Apr 01, 2025 · 6 min read
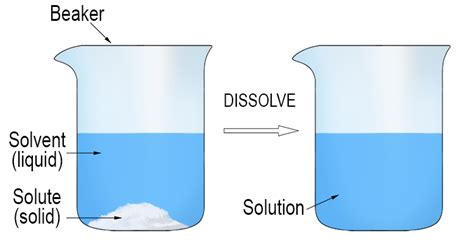
Table of Contents
A Deep Dive into Solutes: The Dissolved Components of Solutions
Understanding solutions is fundamental to chemistry, biology, and numerous other fields. A solution, simply put, is a homogeneous mixture of two or more substances. Crucially, within this mixture, one substance dissolves into another. This dissolved substance is known as the solute, and the substance it dissolves into is called the solvent. This article will delve into the fascinating world of solutes, exploring their properties, behaviors, and importance across various disciplines.
What is a Solute?
A solute is any substance that dissolves in a solvent to form a solution. It can exist in various forms, including solids, liquids, or gases. The key characteristic of a solute is its ability to disperse uniformly throughout the solvent at a molecular or ionic level, resulting in a homogeneous mixture where the solute is no longer visibly distinct. Think of dissolving sugar (the solute) in water (the solvent); the sugar disappears, becoming indistinguishable from the water, creating a sugar solution.
Types of Solutes: A Diverse Range
Solutes are incredibly diverse and span a wide range of chemical compounds and elements. We can categorize them based on several criteria:
1. Based on Chemical Nature:
-
Ionic Solutes: These solutes dissociate into ions when dissolved in a solvent, typically polar solvents like water. Table salt (NaCl) is a classic example. When dissolved in water, it breaks down into sodium (Na+) and chloride (Cl-) ions. The strong electrostatic interactions between these ions and water molecules facilitate the dissolution process.
-
Molecular Solutes: These solutes dissolve without dissociating into ions. Sugar (sucrose), for instance, dissolves in water as individual molecules, maintaining its molecular structure. The dissolution is driven by interactions between the polar sugar molecules and water molecules.
-
Metallic Solutes: While less common in typical solutions, certain metals can dissolve in liquid metals, forming alloys. This process is often driven by the metallic bonding characteristics of both the solute and solvent.
2. Based on Solubility:
Solubility describes the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. This property significantly influences the behavior of the solution.
-
Highly Soluble Solutes: These solutes dissolve readily in a solvent, often in large quantities. Many salts and sugars fall into this category.
-
Slightly Soluble Solutes: These solutes dissolve to a limited extent in the solvent. The solubility may be influenced by factors like temperature or the presence of other substances.
-
Insoluble Solutes: These solutes essentially do not dissolve in the given solvent, even at high concentrations. Sand in water is a good example.
3. Based on Polarity:
Polarity plays a crucial role in determining solute solubility.
-
Polar Solutes: Polar solutes dissolve readily in polar solvents (like water) due to strong solute-solvent interactions. The principle "like dissolves like" perfectly encapsulates this behavior.
-
Nonpolar Solutes: Nonpolar solutes tend to dissolve best in nonpolar solvents (like oil). These solutes interact through weaker van der Waals forces.
The Dissolution Process: A Microscopic View
The dissolution of a solute is not a passive process; it involves intricate interactions at the molecular level. The steps involved generally include:
-
Solvent-Solute Interaction: The solvent molecules approach the solute particles (ions or molecules).
-
Breaking of Intermolecular Forces: The solvent molecules must overcome the attractive forces holding the solute particles together (e.g., ionic bonds, hydrogen bonds, van der Waals forces). This requires energy.
-
Solvation: Once the solute particles are separated, they become surrounded by solvent molecules. This process, called solvation (or hydration in the case of water as the solvent), stabilizes the solute particles in the solution and prevents them from re-aggregating.
-
Uniform Dispersion: The solute particles are uniformly distributed throughout the solvent, forming a homogeneous solution.
Factors Affecting Solute Solubility
Several factors influence how much solute can dissolve in a given solvent:
-
Temperature: For most solid solutes, solubility increases with increasing temperature. However, this is not always the case; some solutes exhibit decreased solubility with increasing temperature. The solubility of gases generally decreases with increasing temperature.
-
Pressure: Pressure significantly affects the solubility of gases, following Henry's Law. Increased pressure increases the solubility of a gas in a liquid. The effect of pressure on solid or liquid solutes is usually negligible.
-
Nature of Solute and Solvent: The "like dissolves like" rule is paramount here. Polar solutes dissolve in polar solvents, and nonpolar solutes dissolve in nonpolar solvents.
-
Presence of Other Substances: The presence of other solutes or impurities in the solvent can alter the solubility of a particular solute. This is often due to interactions between the different solutes and solvent molecules.
Importance of Solutes Across Disciplines
Solutes are central to many processes and applications across various fields:
1. Biology:
-
Electrolyte Balance: Ions (such as sodium, potassium, chloride) dissolved in bodily fluids are crucial for maintaining proper electrolyte balance, nerve impulse transmission, and muscle function.
-
Nutrient Transport: Nutrients are transported throughout the body as dissolved solutes in the bloodstream.
-
Enzyme Activity: Many enzymes require specific solutes to function optimally.
-
Osmosis and Cell Function: The concentration of solutes inside and outside cells determines osmotic pressure and influences cell volume and function.
2. Chemistry:
-
Chemical Reactions: Many chemical reactions occur in solution, with solutes as reactants or products.
-
Analytical Chemistry: Many analytical techniques, such as titration and spectrophotometry, rely on the properties of solutes in solution.
-
Synthesis and Purification: Dissolving and recrystallizing solutes are crucial steps in chemical synthesis and purification processes.
3. Environmental Science:
-
Water Pollution: Dissolved pollutants, such as heavy metals and pesticides, pose serious environmental threats.
-
Ocean Chemistry: The salinity of oceans is determined by the concentration of dissolved salts and minerals.
-
Atmospheric Chemistry: Gases dissolved in rainwater contribute to acid rain.
4. Medicine and Pharmacy:
-
Drug Delivery: Many drugs are administered as solutions, allowing for efficient absorption and distribution throughout the body.
-
Intravenous Fluids: Intravenous fluids contain dissolved solutes that maintain proper fluid and electrolyte balance in patients.
Conclusion: The Unsung Heroes of Solutions
Solutes are the unsung heroes of countless natural processes and technological applications. Their properties and behavior are fundamental to understanding and manipulating the world around us, from the intricacies of biological systems to the complexities of chemical reactions and environmental processes. This article provides a comprehensive overview of solutes, emphasizing their diverse nature, the factors influencing their solubility, and their profound importance in various scientific disciplines. A deeper understanding of solutes allows for innovation in diverse fields, leading to advancements in medicine, environmental protection, and materials science. The seemingly simple act of dissolving a substance holds a wealth of scientific complexity and far-reaching consequences.
Latest Posts
Latest Posts
-
Distance From Earth To Mars In Light Years
Apr 02, 2025
-
Ratio Of Moles Of Water To Moles Of Hydrate
Apr 02, 2025
-
How Many Revolutions Are In A Radian
Apr 02, 2025
-
The Si Unit For Energy Is
Apr 02, 2025
-
Finding The Angle Between Two Planes
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Substance That Is Dissolved In A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
