How To Write Electron Configuration For Ions
listenit
Mar 25, 2025 · 7 min read
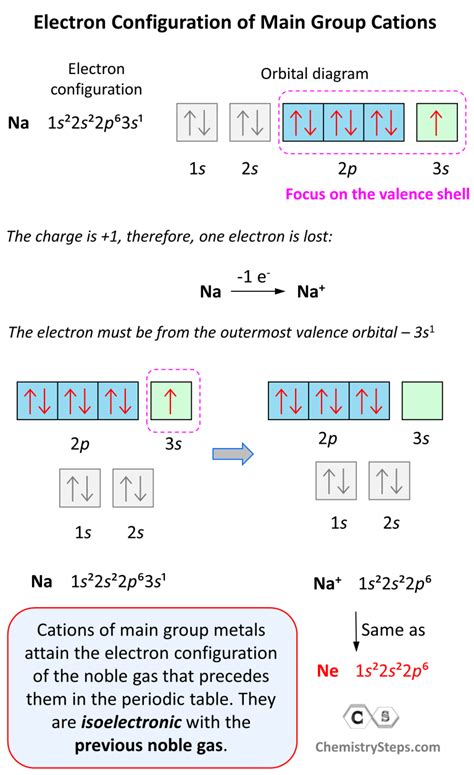
Table of Contents
How to Write Electron Configurations for Ions
Understanding electron configuration is fundamental to chemistry. It describes the arrangement of electrons within an atom's orbitals, dictating its chemical properties and reactivity. While mastering neutral atom configurations is a crucial first step, the ability to write electron configurations for ions is equally important, as ions are central to numerous chemical processes. This comprehensive guide will walk you through the intricacies of determining electron configurations for both cations (positive ions) and anions (negative ions), equipping you with the skills to tackle diverse examples.
Understanding Basic Electron Configuration
Before diving into ions, let's briefly review the rules governing electron configuration for neutral atoms. We use the Aufbau principle, Hund's rule, and the Pauli exclusion principle as our guiding principles.
The Aufbau Principle
The Aufbau principle states that electrons fill orbitals starting from the lowest energy level and progressing upwards. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... Remember the mnemonic devices like "so please stop pulling such darting pins, some fine doctor provided sugar for desserts" to help memorize this order.
Hund's Rule
Hund's rule dictates that electrons individually occupy each orbital within a subshell before pairing up. This minimizes electron-electron repulsion, leading to greater stability.
The Pauli Exclusion Principle
The Pauli exclusion principle states that a maximum of two electrons can occupy a single orbital, and these two electrons must have opposite spins (one spin-up, one spin-down).
Example: Neutral Atom Electron Configuration
Let's consider the element oxygen (O), atomic number 8. This means it has 8 electrons. Following the Aufbau principle, its electron configuration is: 1s²2s²2p⁴. Note that the superscripts indicate the number of electrons in each subshell. Hund's rule is applied to the 2p subshell; the four electrons occupy three separate orbitals, with two orbitals singly occupied and one doubly occupied.
Writing Electron Configurations for Cations (Positive Ions)
Cations form when an atom loses electrons. The number of electrons lost is equal to the cation's charge. When writing the electron configuration of a cation, we remove electrons from the highest energy level (outermost shell) first.
Step-by-Step Guide for Cations
-
Determine the neutral atom's electron configuration: Begin by writing the electron configuration for the neutral atom.
-
Identify the charge of the cation: The charge indicates the number of electrons to remove. For example, Fe²⁺ indicates the loss of two electrons.
-
Remove electrons from the highest energy level: Start by removing electrons from the outermost shell (highest principal quantum number, n). If necessary, remove electrons from the next highest energy level. Always remove electrons from the p subshell before the s subshell within the same principal quantum number if necessary.
Examples of Cation Electron Configurations
-
Sodium ion (Na⁺): Neutral sodium (Na) has an electron configuration of 1s²2s²2p⁶3s¹. To form Na⁺, it loses one electron from the 3s orbital. Therefore, the electron configuration of Na⁺ is 1s²2s²2p⁶.
-
Iron(II) ion (Fe²⁺): Neutral iron (Fe) has an electron configuration of [Ar]3d⁶4s². To form Fe²⁺, it loses two electrons. Following the rule of removing from the highest energy level, we remove the two electrons from the 4s orbital first. Therefore, the electron configuration of Fe²⁺ is [Ar]3d⁶.
-
Iron(III) ion (Fe³⁺): To form Fe³⁺, iron loses three electrons. We remove two electrons from the 4s orbital and one electron from the 3d orbital. The electron configuration of Fe³⁺ is [Ar]3d⁵.
-
Copper(II) ion (Cu²⁺): Neutral copper (Cu) exhibits an anomalous electron configuration of [Ar]3d¹⁰4s¹. To form Cu²⁺, it loses one electron from the 4s orbital and one from the 3d orbital, resulting in [Ar]3d⁹. This exception highlights the importance of understanding electron configuration anomalies.
Writing Electron Configurations for Anions (Negative Ions)
Anions form when an atom gains electrons. The number of electrons gained is equal to the anion's charge. When writing the electron configuration of an anion, we add electrons to the highest energy level that is not fully occupied.
Step-by-Step Guide for Anions
-
Determine the neutral atom's electron configuration: Start by writing the electron configuration of the neutral atom.
-
Identify the charge of the anion: The charge indicates the number of electrons to add. For example, O²⁻ indicates the gain of two electrons.
-
Add electrons to the highest available energy level: Add electrons to the outermost shell, filling subshells according to the Aufbau principle and Hund's rule.
Examples of Anion Electron Configurations
-
Chloride ion (Cl⁻): Neutral chlorine (Cl) has an electron configuration of [Ne]3s²3p⁵. To form Cl⁻, it gains one electron, filling the 3p subshell. The electron configuration of Cl⁻ is [Ne]3s²3p⁶, which is also the electron configuration of Argon (Ar).
-
Oxide ion (O²⁻): Neutral oxygen (O) has an electron configuration of 1s²2s²2p⁴. To form O²⁻, it gains two electrons, completing the 2p subshell. The electron configuration of O²⁻ is 1s²2s²2p⁶, also the electron configuration of Neon (Ne).
-
Sulfide ion (S²⁻): Neutral sulfur (S) has an electron configuration of [Ne]3s²3p⁴. To form S²⁻, it gains two electrons, filling the 3p subshell. The electron configuration of S²⁻ is [Ne]3s²3p⁶.
Dealing with Transition Metal Ions and Exceptions
Transition metals often exhibit exceptions to the standard Aufbau principle due to the relatively close energy levels of the (n-1)d and ns orbitals. This can lead to unexpected electron configurations for both neutral atoms and their ions.
Remember that the energy levels are not always perfectly ordered as predicted. Sometimes, a half-filled or completely filled d subshell is more stable than a configuration that would otherwise be predicted based on the simple filling order. This is especially true in transition metal ions. The most stable configurations usually involve the half-filled or fully filled d orbitals.
For example, chromium (Cr) has an electron configuration of [Ar]3d⁵4s¹, not [Ar]3d⁴4s², because a half-filled 3d subshell is more stable. Similarly, copper (Cu) has an electron configuration of [Ar]3d¹⁰4s¹, not [Ar]3d⁹4s², because a completely filled 3d subshell is more stable. These exceptions must be considered when determining the electron configurations of their ions.
Remember to always prioritize removing electrons from the highest energy level first when creating cation configurations.
Practical Applications and Importance
The ability to accurately predict the electron configuration of ions is crucial in various fields:
-
Chemistry: Understanding ion configurations helps predict chemical bonding, reactivity, and the formation of compounds. It plays a significant role in understanding redox reactions, where electron transfer is central.
-
Materials Science: The electron configuration of ions in materials dictates their electrical conductivity, magnetic properties, and optical behavior. This is critical in designing new materials with specific properties.
-
Biochemistry: Many biological processes rely on metal ions with specific electron configurations. Understanding these configurations is essential for comprehending enzymatic activity and other biological functions.
Conclusion
Mastering the art of writing electron configurations for ions is a vital skill for any student or professional working in the realm of chemistry and related fields. By understanding the fundamental principles—the Aufbau principle, Hund's rule, and the Pauli exclusion principle—and carefully following the steps outlined above, you can confidently determine the electron configuration of virtually any ion, including those of transition metals with their occasional exceptions. This skill will pave the way for a deeper understanding of chemical bonding, reactivity, and the diverse properties of matter. Regular practice with various examples will solidify your understanding and make this seemingly complex task a straightforward one. Remember to consult periodic tables and reliable chemical resources to verify your answers and enhance your understanding.
Latest Posts
Latest Posts
-
How To Figure Diameter With Circumference
Mar 25, 2025
-
What Is The Square Root Of 245
Mar 25, 2025
-
What Is 1 3 Plus 1 4
Mar 25, 2025
-
The Study Of The Cells Is Called
Mar 25, 2025
-
Can Mixtures Be Separated By Physical Means
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Write Electron Configuration For Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
