How To Find Number Of Molecules
listenit
Mar 21, 2025 · 5 min read
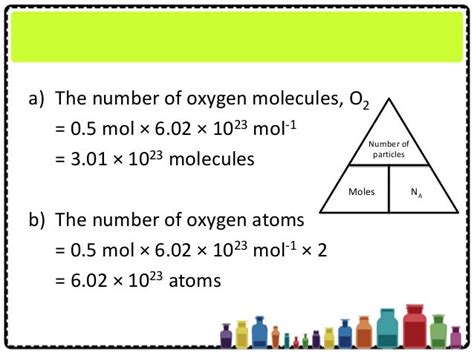
Table of Contents
How to Find the Number of Molecules: A Comprehensive Guide
Determining the number of molecules in a given substance is a fundamental concept in chemistry, crucial for various applications ranging from stoichiometric calculations to understanding reaction yields. This comprehensive guide will explore different methods for calculating the number of molecules, catering to various levels of understanding, from basic introductions to more advanced scenarios.
Understanding the Mole Concept
Before diving into the methods, it's essential to grasp the core concept of the mole (mol). A mole isn't a fuzzy creature; it's a unit representing a specific number of particles, namely Avogadro's number (N<sub>A</sub>), which is approximately 6.022 x 10<sup>23</sup>. One mole of any substance contains Avogadro's number of particles, whether they are atoms, molecules, ions, or formula units. This concept forms the bridge between the macroscopic world (grams, liters) and the microscopic world (atoms, molecules).
Relating Moles to Grams: Molar Mass
The molar mass (M) of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's numerically equal to the atomic or molecular weight of the substance. For example, the molar mass of water (H₂O) is approximately 18 g/mol (2 x 1 g/mol for Hydrogen + 16 g/mol for Oxygen).
The Key Equation: Linking Moles, Mass, and Avogadro's Number
The fundamental equation linking moles, mass, and Avogadro's number is:
Number of molecules = (Mass of substance / Molar mass) x Avogadro's number
This equation provides the primary method for calculating the number of molecules. Let's break down how to use it step-by-step with examples.
Method 1: Calculating the Number of Molecules from Mass
This is the most common method, applicable when you know the mass of the substance.
Step 1: Determine the Molar Mass
Find the molar mass of the substance using the periodic table. Add the atomic masses of all atoms in the molecule. For example:
- Water (H₂O): 2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
- Carbon Dioxide (CO₂): 12.01 g/mol + 2(16.00 g/mol) = 44.01 g/mol
- Glucose (C₆H₁₂O₆): 6(12.01 g/mol) + 12(1.01 g/mol) + 6(16.00 g/mol) = 180.18 g/mol
Step 2: Convert Mass to Moles
Divide the given mass of the substance by its molar mass. Remember to use consistent units (grams for mass and g/mol for molar mass).
Example: Find the number of moles in 10 grams of water.
Moles of water = 10 g / 18.02 g/mol ≈ 0.555 moles
Step 3: Calculate the Number of Molecules
Multiply the number of moles by Avogadro's number (6.022 x 10<sup>23</sup> molecules/mol).
Number of water molecules = 0.555 moles x 6.022 x 10<sup>23</sup> molecules/mol ≈ 3.34 x 10<sup>23</sup> molecules
Method 2: Calculating the Number of Molecules from Volume (Gases)
For gases, you can use the Ideal Gas Law to find the number of moles and then calculate the number of molecules.
The Ideal Gas Law is: PV = nRT
Where:
- P = Pressure (usually in atmospheres, atm)
- V = Volume (usually in liters, L)
- n = Number of moles
- R = Ideal gas constant (0.0821 L·atm/mol·K)
- T = Temperature (in Kelvin, K)
Step 1: Solve for n (moles)
Rearrange the Ideal Gas Law to solve for n: n = PV/RT
Step 2: Calculate the Number of Molecules
Once you have the number of moles (n), multiply it by Avogadro's number to get the number of molecules.
Example: A gas occupies 5 liters at 25°C and 1 atm pressure. Find the number of molecules.
First, convert the temperature to Kelvin: 25°C + 273.15 = 298.15 K
Then, calculate the number of moles: n = (1 atm x 5 L) / (0.0821 L·atm/mol·K x 298.15 K) ≈ 0.204 moles
Finally, calculate the number of molecules: 0.204 moles x 6.022 x 10<sup>23</sup> molecules/mol ≈ 1.23 x 10<sup>23</sup> molecules
Method 3: Calculating Number of Molecules from Concentration (Solutions)
For solutions, you need to know the concentration (usually in molarity, M) and volume.
Molarity (M) = Moles of solute / Liters of solution
Step 1: Calculate Moles of Solute
Rearrange the molarity equation to find moles: Moles of solute = Molarity x Liters of solution
Step 2: Calculate the Number of Molecules
Multiply the number of moles by Avogadro's number.
Example: A 0.1 M solution of NaCl has a volume of 250 mL. Find the number of NaCl formula units.
First, convert mL to L: 250 mL = 0.250 L
Next, calculate the moles of NaCl: 0.1 M x 0.250 L = 0.025 moles
Finally, calculate the number of formula units: 0.025 moles x 6.022 x 10<sup>23</sup> formula units/mol ≈ 1.51 x 10<sup>22</sup> formula units (Note: NaCl is an ionic compound, so we use formula units instead of molecules).
Advanced Scenarios and Considerations
-
Dealing with Impurities: If your sample contains impurities, you'll need to account for the percentage purity. For example, if a sample is 95% pure, you would multiply the calculated number of molecules by 0.95.
-
Using Avogadro's Law: This law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. It can be useful in comparing the number of molecules in different gases under identical conditions.
-
Dealing with Mixtures: For mixtures, calculate the number of molecules for each component separately and then add them together.
-
Limitations of the Ideal Gas Law: The Ideal Gas Law is an approximation, and it works best for gases at low pressures and high temperatures. For gases under extreme conditions, more complex equations of state are needed.
Conclusion: Mastering Molecular Calculations
Calculating the number of molecules is a fundamental skill in chemistry. This guide provides a clear path, from basic concepts to more complex scenarios, empowering you to confidently tackle a wide range of problems involving molecular quantities. Remember to pay close attention to units and always double-check your calculations to ensure accuracy. With practice, you’ll become proficient in translating macroscopic measurements into the microscopic world of molecules. By understanding the interplay between mass, moles, and Avogadro's number, you’ll gain a deeper appreciation for the quantitative nature of chemistry and its ability to explain the world around us at its most fundamental level.
Latest Posts
Latest Posts
-
How To Find Ph Of Buffer Solution
Mar 27, 2025
-
5 Less Than The Product Of 3 And A Number
Mar 27, 2025
-
Where Is Most Of Earths Freshwater Stored
Mar 27, 2025
-
How Many Protons Does Strontium Have
Mar 27, 2025
-
Is The Earth Older Than The Sun
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Find Number Of Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
