How To Find Charge Of Transition Metals
listenit
Mar 23, 2025 · 6 min read
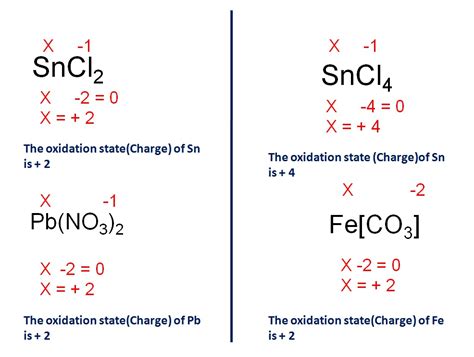
Table of Contents
How to Find the Charge of Transition Metals: A Comprehensive Guide
Transition metals, elements found in the d-block of the periodic table, are renowned for their variable oxidation states. Unlike alkali metals or halogens with predictable charges, determining the charge of a transition metal ion requires a deeper understanding of its chemical environment and the principles governing its bonding. This comprehensive guide delves into various methods and strategies to effectively ascertain the charge of transition metals in different compounds.
Understanding Transition Metal Chemistry
Before diving into specific techniques, it's crucial to establish a foundational understanding of transition metal chemistry. The unique properties of these elements stem from their partially filled d orbitals, allowing them to exhibit multiple oxidation states. This variability is a key factor influencing their diverse roles in biological systems and industrial applications. The ability to readily gain or lose electrons contributes to their versatile reactivity.
Variable Oxidation States: The Core Challenge
The primary challenge in determining the charge of a transition metal lies in its capacity to exist in multiple oxidation states. For instance, iron (Fe) can exist as Fe²⁺ (ferrous) or Fe³⁺ (ferric), drastically altering its chemical behavior and properties. This variability is not only dependent on the metal itself but also heavily influenced by the ligands (atoms, ions, or molecules surrounding the metal center) and the overall chemical environment.
The Role of Ligands
Ligands play a crucial role in stabilizing different oxidation states of transition metals. Different ligands have different strengths in stabilizing specific oxidation states. Strong-field ligands, for example, can lead to higher oxidation states, while weak-field ligands may favor lower oxidation states. The nature of the ligand-metal bond significantly impacts the electronic configuration and thus the overall charge on the metal ion. Understanding ligand field theory is essential for predicting the charge of a transition metal.
Methods for Determining the Charge of Transition Metals
Several methods can be employed to determine the charge of a transition metal ion within a compound. These methods range from simple stoichiometric calculations to more advanced spectroscopic techniques.
1. Stoichiometry and Charge Balance
This is the most fundamental approach, relying on the principle of charge neutrality in a compound. By knowing the charges of the other ions or ligands present, we can deduce the charge of the transition metal.
Example: Consider the compound FeCl₃. We know that chlorine (Cl) has a -1 charge. To maintain charge neutrality in the compound, the total positive charge must equal the total negative charge. Since there are three chloride ions, the total negative charge is -3. Therefore, the iron ion (Fe) must have a +3 charge to balance this, indicating Fe³⁺.
Limitations: This method is straightforward for simple ionic compounds but becomes less reliable for complex coordination compounds where the metal is bound to multiple ligands with different charges or when the compound contains counter ions.
2. Oxidation State Determination through Reaction Analysis
By observing the reaction a compound undergoes, we can sometimes infer the oxidation state of the transition metal. Redox reactions, in particular, provide clues. The change in the oxidation state of the transition metal during a reaction can be used to determine its initial oxidation state.
Example: If a compound containing a transition metal undergoes a reduction reaction where it gains two electrons, and we know the final oxidation state, we can determine the initial oxidation state by adding two to the final oxidation state.
Limitations: Requires understanding of redox reactions and their stoichiometry. The method becomes challenging when multiple oxidation states are possible or when the reaction pathway is not straightforward.
3. Spectroscopic Techniques
Spectroscopic techniques offer powerful tools for directly probing the electronic structure of transition metal complexes, providing insights into their oxidation states. Several spectroscopic methods are particularly useful:
-
UV-Vis Spectroscopy: Transition metal complexes often exhibit characteristic absorption bands in the ultraviolet-visible (UV-Vis) region of the electromagnetic spectrum. The position and intensity of these bands are highly sensitive to the oxidation state and the ligand field environment of the metal ion. Analysis of the UV-Vis spectrum can provide valuable information about the oxidation state.
-
X-ray Photoelectron Spectroscopy (XPS): XPS measures the binding energies of core electrons, which are sensitive to the oxidation state of the atom. By analyzing the core level spectra, the oxidation state of the transition metal can be determined with high accuracy.
-
Electron Paramagnetic Resonance (EPR) Spectroscopy: EPR spectroscopy is particularly useful for studying paramagnetic species, which have unpaired electrons. Many transition metal ions in certain oxidation states exhibit paramagnetism. The EPR spectrum provides detailed information about the electronic structure and hence the oxidation state.
-
Mössbauer Spectroscopy: This technique is particularly valuable for studying iron compounds, providing sensitive information about the oxidation state, spin state, and coordination environment of iron atoms.
Limitations: Spectroscopic techniques often require specialized equipment and expertise in data interpretation. The cost of the equipment can also be a limiting factor.
4. Magnetic Susceptibility Measurements
Magnetic susceptibility measurements can reveal whether a compound is paramagnetic (possessing unpaired electrons) or diamagnetic (no unpaired electrons). Many transition metal ions in specific oxidation states exhibit paramagnetism due to unpaired electrons in their d orbitals. Measuring the magnetic susceptibility can indicate the presence of unpaired electrons and indirectly suggest a possible oxidation state.
Limitations: This method doesn't provide a definitive oxidation state but rather offers clues consistent with certain oxidation states. It is less precise than spectroscopic techniques.
5. Electrochemical Methods
Electrochemical methods, such as cyclic voltammetry, can be employed to determine the redox potentials of transition metal complexes. The redox potentials provide information about the relative stability of different oxidation states. By analyzing the electrochemical behavior, one can infer the most likely oxidation state under specific conditions.
Limitations: These methods require specialized electrochemical equipment and a good understanding of electrochemical principles.
Advanced Considerations and Complex Scenarios
Determining the charge of transition metals becomes more complex in scenarios involving:
-
Mixed Oxidation States: Some compounds contain transition metals in multiple oxidation states simultaneously. Advanced techniques like X-ray absorption spectroscopy (XAS) may be necessary to differentiate and quantify these states.
-
Cluster Compounds: In cluster compounds, multiple transition metal atoms are bonded together, making charge assignment challenging. Careful analysis of the bonding interactions and overall charge balance is crucial.
-
Organometallic Complexes: Organometallic compounds involve metal-carbon bonds, adding further complexity to the charge assignment. A combination of spectroscopic techniques and theoretical calculations may be required.
Conclusion
Determining the charge of transition metals requires a multifaceted approach, utilizing various techniques and combining knowledge from different aspects of chemistry. Stoichiometric calculations provide a basic starting point, but spectroscopic and electrochemical methods offer higher precision and detailed information about the electronic structure. The selection of the most appropriate method depends on the complexity of the compound, the available resources, and the desired level of accuracy. A thorough understanding of transition metal chemistry, ligand field theory, and redox reactions is essential for accurately interpreting the data and determining the charge of these versatile elements. By mastering these techniques and employing a critical approach to data interpretation, one can successfully unravel the intricate charge behavior of transition metals.
Latest Posts
Latest Posts
-
Where Is Absolute Value On Ti 84
Mar 25, 2025
-
Which Element Has A Higher Ionization Energy
Mar 25, 2025
-
1 8 Of A Yard Is How Many Inches
Mar 25, 2025
-
7 3 5 As An Improper Fraction
Mar 25, 2025
-
Net Ionic Equation For Hcl Naoh
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Find Charge Of Transition Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
