Which Element Has A Higher Ionization Energy
listenit
Mar 25, 2025 · 6 min read
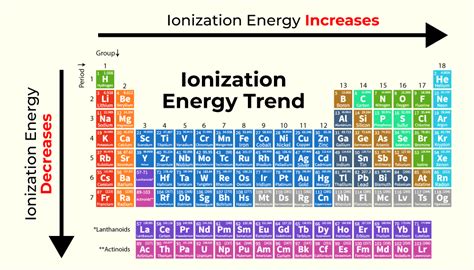
Table of Contents
Which Element Has a Higher Ionization Energy? A Deep Dive into Atomic Structure and Periodic Trends
Ionization energy, a fundamental concept in chemistry and physics, measures the energy required to remove an electron from a gaseous atom or ion. Understanding which element boasts a higher ionization energy is crucial for comprehending atomic structure, periodic trends, and the behavior of elements in chemical reactions. This comprehensive guide delves into the intricacies of ionization energy, exploring the factors influencing it and examining specific examples to illustrate the underlying principles.
Understanding Ionization Energy: The Basics
Ionization energy isn't a single value; rather, it's a series of values, each representing the energy needed to remove successive electrons. The first ionization energy (IE<sub>1</sub>) is the energy required to remove the outermost electron, the second ionization energy (IE<sub>2</sub>) removes the next electron, and so on. These successive ionization energies generally increase, as removing each electron leaves a more positively charged ion, making it progressively harder to remove subsequent electrons. The magnitude of these energies provides insights into the element's electronic configuration and its position within the periodic table.
Factors Affecting Ionization Energy:
Several key factors influence an element's ionization energy:
1. Atomic Radius: The Distance Matters
Smaller atoms generally exhibit higher ionization energies. This is because the outermost electrons are closer to the positively charged nucleus, experiencing a stronger electrostatic attraction. The stronger the attraction, the more energy is required to overcome it and remove the electron. As you move across a period in the periodic table (left to right), atomic radius decreases, leading to a corresponding increase in ionization energy.
2. Nuclear Charge: The Pull of the Nucleus
The number of protons in the nucleus (atomic number) directly impacts the strength of the electrostatic attraction between the nucleus and the electrons. A higher nuclear charge results in a stronger pull on the electrons, increasing the ionization energy. This effect is primarily observed when comparing elements within the same period.
3. Shielding Effect: Inner Electrons' Role
Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons. Elements with more inner electrons exhibit a greater shielding effect, resulting in lower ionization energies. The shielding effect is particularly significant when comparing elements within the same group (column) of the periodic table.
4. Electron Configuration: Stability and Subshells
The electron configuration of an atom influences its ionization energy. Elements with stable electron configurations (e.g., noble gases with filled electron shells) have exceptionally high ionization energies because removing an electron disrupts this stability. Half-filled and fully-filled subshells also exhibit relatively high ionization energies due to enhanced stability.
Periodic Trends in Ionization Energy: A Visual Guide
The periodic table provides a powerful tool for understanding and predicting ionization energy trends.
1. Across a Period (Left to Right): The Increase
As you move across a period from left to right, ionization energy generally increases. This is primarily due to the increasing nuclear charge and decreasing atomic radius. The increased nuclear charge pulls the outermost electrons more strongly towards the nucleus, while the smaller atomic radius reduces the distance between the nucleus and the electrons, resulting in a stronger electrostatic attraction.
Example: Consider the second period: Lithium (Li) has a lower ionization energy than beryllium (Be), which in turn has a lower ionization energy than boron (B), and so on, until you reach neon (Ne), which has the highest ionization energy in the period.
2. Down a Group (Top to Bottom): The Decrease
As you move down a group from top to bottom, ionization energy generally decreases. This trend is attributed to the increasing atomic radius and increased shielding effect. The larger atomic radius increases the distance between the nucleus and the outermost electrons, weakening the electrostatic attraction. Furthermore, the increased number of inner electrons enhances the shielding effect, further reducing the effective nuclear charge experienced by the outermost electrons.
Example: Consider Group 1 (alkali metals): Lithium (Li) has a higher ionization energy than sodium (Na), which has a higher ionization energy than potassium (K), and so on.
Exceptions to the General Trends
While the general trends are predictable, there are exceptions. These exceptions often arise from variations in electron configurations and subshell stability. For instance:
-
Between Groups IIA and IIIA: The ionization energy sometimes decreases slightly going from group IIA to IIIA. This is because the third electron in group IIIA elements occupies a higher energy level (p-orbital) that experiences a greater shielding effect compared to the s-orbital electrons in group IIA.
-
Between Groups VA and VIA: A similar slight decrease can be observed between groups VA and VIA. The added electron in group VIA occupies the same p-subshell as the other electrons resulting in increased electron-electron repulsion, making it slightly easier to remove an electron.
Comparing Specific Elements: Case Studies
Let's consider some specific examples to illustrate the concepts discussed:
Helium (He) vs. Hydrogen (H): Helium, with its filled 1s<sup>2</sup> electron shell, has a significantly higher first ionization energy than hydrogen. The increased nuclear charge and greater stability of the filled shell contribute to this difference.
Fluorine (F) vs. Neon (Ne): Fluorine has a higher first ionization energy than Neon despite neon's higher atomic number. This is because fluorine has one less electron in a completely filled shell. Adding an electron to a higher shell causes greater repulsion hence lower Ionization energy.
Oxygen (O) vs. Nitrogen (N): Although nitrogen has a higher atomic number than oxygen, oxygen has a slightly higher first ionization energy. This is because nitrogen has a half-filled p subshell (p<sup>3</sup>), which is relatively stable. Removing an electron from this stable configuration requires more energy.
Sodium (Na) vs. Magnesium (Mg): Magnesium has a higher first ionization energy than sodium due to its higher nuclear charge. The effect of the additional proton outweighs the slight increase in electron-electron repulsion.
Applications of Ionization Energy: Real-World Significance
Understanding ionization energy is crucial in several fields:
-
Chemistry: It's essential for predicting reactivity, bond formation, and chemical behavior. Elements with low ionization energies readily lose electrons and form cations, while those with high ionization energies tend to gain electrons and form anions.
-
Physics: Ionization energy is vital in understanding atomic spectra, plasma physics, and the behavior of matter under extreme conditions.
-
Materials Science: Ionization energy influences material properties, including electrical conductivity, thermal conductivity, and reactivity with other materials.
Conclusion: A Powerful Tool for Understanding the Atom
Ionization energy serves as a powerful tool for understanding the fundamental properties of elements and their interactions. By considering atomic radius, nuclear charge, shielding effect, and electron configuration, we can effectively predict and explain trends in ionization energy across the periodic table. Furthermore, a thorough understanding of these trends is essential for comprehending a vast range of chemical and physical phenomena, from everyday chemical reactions to advanced scientific applications. The more we delve into the intricacies of ionization energy, the deeper our understanding of the atomic world becomes.
Latest Posts
Latest Posts
-
Is Sugar A Compound Or A Mixture
Mar 25, 2025
-
What Is The Reactant In Photosynthesis
Mar 25, 2025
-
Which Expression Represents The Width Of The Framed Picture
Mar 25, 2025
-
What Is A Prime Factorization Of 44
Mar 25, 2025
-
Quadrilateral With 2 Sets Of Parallel Sides
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has A Higher Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
