Net Ionic Equation For Hcl + Naoh
listenit
Mar 25, 2025 · 5 min read
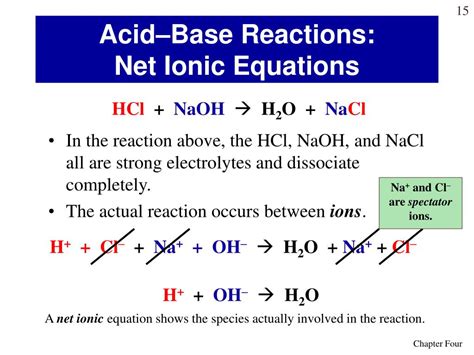
Table of Contents
Net Ionic Equation for HCl + NaOH: A Deep Dive into Acid-Base Reactions
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a strong acid-strong base neutralization reaction. Understanding this reaction, especially at the level of the net ionic equation, is crucial for grasping fundamental concepts in chemistry, particularly acid-base chemistry and stoichiometry. This comprehensive guide will delve into the intricacies of this reaction, explaining the process step-by-step and highlighting its significance in various chemical contexts.
Understanding the Reactants: HCl and NaOH
Before diving into the net ionic equation, let's establish a firm understanding of the individual reactants:
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid, meaning it completely dissociates into its ions (H⁺ and Cl⁻) when dissolved in water. This complete dissociation is key to understanding its behavior in reactions. The equation for this dissociation is:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The (aq) notation indicates that the species are dissolved in an aqueous solution (water). The complete dissociation of HCl means that in solution, there are virtually no undissociated HCl molecules present.
Sodium Hydroxide (NaOH)
Sodium hydroxide is a strong base, similarly undergoing complete dissociation in water. The dissociation equation is:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Like HCl, NaOH's complete dissociation means that in solution, we primarily have its constituent ions, Na⁺ and OH⁻, rather than intact NaOH molecules.
The Complete Ionic Equation
Now, let's combine the dissociated ions of HCl and NaOH to represent the complete ionic equation for the reaction:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → ?
This equation shows all the ions present in the solution before the reaction takes place. To complete the equation, we need to consider the products formed.
The Products: Water and Salt
The reaction between HCl and NaOH is a neutralization reaction, resulting in the formation of water (H₂O) and a salt. In this case, the salt is sodium chloride (NaCl). The balanced molecular equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Notice that the water is indicated as (l), representing it as a liquid. The sodium chloride remains dissolved in the aqueous solution.
Constructing the Complete Ionic Equation
To write the complete ionic equation, we replace the molecular formulas of HCl and NaOH with their dissociated ions, reflecting the complete dissociation in solution:
H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation explicitly shows all the ions present before and after the reaction.
Deriving the Net Ionic Equation
The net ionic equation focuses on the species that actually participate in the chemical change. In this case, we observe that Na⁺ and Cl⁻ ions are present on both sides of the complete ionic equation. These ions are spectator ions, meaning they do not directly participate in the reaction; they simply remain dissolved in the solution. To obtain the net ionic equation, we cancel out these spectator ions:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This is the net ionic equation for the reaction between HCl and NaOH. It concisely represents the essence of the neutralization reaction: the combination of hydrogen ions (H⁺) and hydroxide ions (OH⁻) to form water (H₂O).
Significance and Applications of the Net Ionic Equation
The net ionic equation provides crucial insights into the chemical process:
- Simplicity and Clarity: It simplifies the representation of the reaction by focusing on the key species involved, eliminating the unnecessary spectator ions. This enhances understanding and allows for easier analysis.
- Predicting Reactions: The net ionic equation helps predict the outcome of similar acid-base reactions involving strong acids and strong bases. Any strong acid reacting with any strong base will always lead to the same net ionic equation.
- Stoichiometric Calculations: The net ionic equation forms the basis for stoichiometric calculations, helping determine the amounts of reactants and products involved in the reaction.
- Understanding Solution Chemistry: It provides a clear picture of the ionic interactions occurring in the solution, clarifying how ions interact and recombine.
Further Exploration: Weak Acids and Bases
It is important to note that the net ionic equation approach described above is specifically for strong acids and strong bases. When dealing with weak acids or weak bases, the situation becomes more complex because these substances do not fully dissociate in solution. Their equilibrium dissociation needs to be considered, leading to more intricate net ionic equations.
Practical Applications of HCl and NaOH Reaction
The neutralization reaction between HCl and NaOH has numerous practical applications:
- Titration: This reaction is fundamental in titrations, a common laboratory technique used to determine the concentration of an unknown solution. By carefully adding a known concentration of HCl (or NaOH) to a solution of NaOH (or HCl), the unknown concentration can be precisely determined.
- Acid Spills: NaOH solutions are sometimes used to neutralize accidental spills of HCl, converting the corrosive acid into relatively harmless salt and water.
- Industrial Processes: The reaction plays a role in various industrial processes where precise pH control is critical.
- Chemical Synthesis: This reaction is a crucial step in many chemical syntheses where specific pH conditions are required.
Beyond the Basics: Exploring Related Concepts
The HCl + NaOH reaction serves as a springboard to understanding several related concepts:
- pH and pOH: The reaction directly impacts the pH and pOH of the solution. As the strong acid and base react, the pH moves toward neutrality (pH 7).
- Equilibrium Constants: While not directly applicable to the strong acid/strong base scenario, the concepts of equilibrium constants (Kₐ and Kₕ) become relevant when considering weak acid/weak base reactions.
- Thermodynamics: The enthalpy change (ΔH) for this reaction can be determined, providing information about the heat released or absorbed during the process.
- Electrochemistry: The reaction can be explored within an electrochemical context, considering the movement of ions and the potential difference between electrodes.
Conclusion
The net ionic equation for the reaction between HCl and NaOH (H⁺(aq) + OH⁻(aq) → H₂O(l)) is a cornerstone concept in chemistry. Its simplicity belies its profound importance in understanding acid-base reactions, stoichiometry, and solution chemistry. By mastering this fundamental concept, you build a strong foundation for exploring more complex chemical phenomena and applications. The reaction’s practical relevance extends to various scientific and industrial fields, highlighting the importance of understanding its underlying chemistry. Remember to always consider the strength of the acids and bases involved when determining the appropriate net ionic equation, as weak acids and bases will require a different approach.
Latest Posts
Latest Posts
-
Why Is Hf A Weak Acid
Mar 25, 2025
-
A Column Of The Periodic Table Is Called A
Mar 25, 2025
-
Derivative Of Square Root Of 3x
Mar 25, 2025
-
A Quadrilateral With Two Pairs Of Parallel Sides Is A
Mar 25, 2025
-
1 Divided By 3 4 As A Fraction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Hcl + Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
