Why Is Hf A Weak Acid
listenit
Mar 25, 2025 · 5 min read
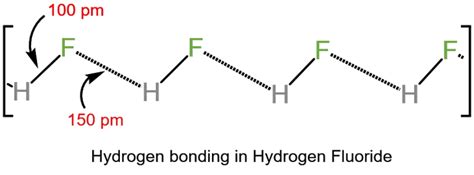
Table of Contents
Why is HF a Weak Acid? A Deep Dive into Hydrofluoric Acid's Behavior
Hydrofluoric acid (HF), despite containing the highly electronegative fluorine atom, is surprisingly a weak acid. This seemingly paradoxical behavior has intrigued chemists for decades, leading to extensive research and a deeper understanding of the factors governing acid strength. This article delves into the reasons behind HF's weak acidity, exploring the intricacies of its structure, bonding, and interactions in solution.
Understanding Acid Strength: A Brief Overview
Before dissecting HF's unique properties, let's establish a foundational understanding of acid strength. An acid's strength is determined by its ability to donate a proton (H⁺) to a base. Strong acids readily donate protons, leading to essentially complete dissociation in water. Conversely, weak acids only partially dissociate, maintaining a significant equilibrium between the undissociated acid and its conjugate base. This equilibrium is quantified by the acid dissociation constant, Ka. A higher Ka value indicates a stronger acid.
The Unexpected Weakness of HF: Contrasting with Other Hydrogen Halides
The hydrogen halides (HF, HCl, HBr, HI) exhibit a fascinating trend in acid strength. HCl, HBr, and HI are all strong acids, completely dissociating in aqueous solutions. However, HF stands out as a weak acid, possessing a significantly lower Ka value than its heavier halogen counterparts. This stark contrast demands an explanation.
The Role of Bond Strength: A Key Factor
One primary reason for HF's weak acidity lies in the exceptionally strong hydrogen-fluorine bond (H-F). The small size and high electronegativity of fluorine lead to a short and strong bond. This strong bond requires a considerable amount of energy to break, hindering the release of a proton. In contrast, the H-Cl, H-Br, and H-I bonds are progressively weaker, making proton donation much easier.
Electronegativity and Bond Polarity: Influence on Acid Strength
While fluorine's high electronegativity contributes to the strong H-F bond, it might seem counterintuitive that this doesn't lead to stronger acidity. The electronegativity difference between hydrogen and fluorine indeed leads to a highly polar H-F bond. However, the strength of the bond itself outweighs the effect of this polarity in determining the ease of proton dissociation. The strong bond holds the proton tightly, even in the presence of a polar solvent like water.
Hydration Effects: Solvent Interactions and Acid Dissociation
The role of the solvent, water, is crucial in understanding acid dissociation. When an acid dissolves in water, the water molecules interact with the resulting ions (H⁺ and the conjugate base), a process called hydration. This hydration stabilizes the ions, promoting dissociation. While the highly electronegative fluorine atom in HF does interact favorably with water molecules through hydrogen bonding, this interaction is not as strong as the stabilization effects that occur with the larger conjugate bases of HCl, HBr, and HI. The smaller size of the fluoride ion (F⁻) leads to a higher charge density, resulting in stronger ion-ion interactions and potentially hindering complete dissociation.
Hydrogen Bonding: A Unique Influence in HF
Hydrogen bonding plays a significant role in HF's behavior in aqueous solution. The highly polar H-F bond allows for strong hydrogen bonding between HF molecules and between HF and water molecules. These hydrogen bonds form extensive networks in solution, effectively hindering the complete dissociation of HF molecules into H⁺ and F⁻ ions. This contrasts with the other hydrogen halides where hydrogen bonding is significantly weaker, less impactful on dissociation.
HF's Behavior in Different Solvents: Beyond Aqueous Solutions
The behavior of HF isn't solely determined by its interaction with water. In other solvents, its acidity can vary depending on the solvent's properties. For instance, in less polar solvents, the dissociation of HF is even less favored due to the reduced stabilization of the ions. This further highlights the impact of solvent interactions on HF's acid strength.
Practical Implications of HF's Weak Acidity
The weak acidity of HF has significant practical implications. While its relatively low dissociation makes it a weaker acid than HCl or HBr, HF is still a highly corrosive substance, capable of etching glass and dissolving many metal oxides. This behavior is a result of the fluoride ion's unique ability to form complexes with silicon and other elements. It's crucial to handle HF with extreme caution due to its potential health hazards.
Comparing HF to other Weak Acids
To further understand HF's position within the landscape of weak acids, let's compare it to some other common examples. While acetic acid (CH₃COOH) and formic acid (HCOOH) are also weak acids, they exhibit different dissociation behaviors due to the structure and electronic effects of their organic functional groups. The differences in Ka values highlight the diverse factors governing weak acid strength, beyond simply bond strength and electronegativity.
Advanced Concepts and Further Research
The study of HF's weak acidity extends beyond simple bond strength and electronegativity considerations. Researchers have employed advanced techniques, such as computational chemistry, to investigate the intricacies of HF's solvation and its interaction with water molecules. These studies provide a more detailed picture of the complex interplay of factors determining HF's behavior in solution.
Conclusion: A Complex interplay of Factors
In conclusion, the weak acidity of HF is not a simple phenomenon but rather a result of a complex interplay of factors. The exceptionally strong H-F bond, the unique role of hydrogen bonding, the effect of hydration, and the interplay with the solvent all contribute to HF's unusual behavior compared to its heavier halogen counterparts. Understanding these factors is crucial for appreciating HF's unique properties and its significance in various chemical processes and industrial applications. Further research continues to unravel the intricate details of HF's behavior, refining our understanding of acid-base chemistry and the complexities of chemical interactions in solution. The apparent paradox of a highly electronegative atom forming a weak acid provides a compelling case study in the nuanced relationships governing chemical behavior.
Latest Posts
Latest Posts
-
Is The Square Root Of 5 Irrational
Mar 26, 2025
-
How Many Cm Is 6 Meters
Mar 26, 2025
-
8 Is What Percent Of 4000
Mar 26, 2025
-
What Is The Properties Of Gases
Mar 26, 2025
-
How To Find The Y Intercept Of A Quadratic Function
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Why Is Hf A Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
