How To Calculate The Mass Of Excess Reactant
listenit
Mar 22, 2025 · 6 min read
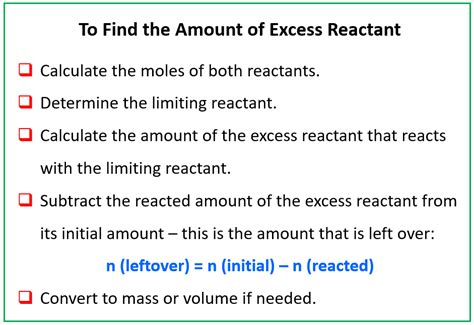
Table of Contents
How to Calculate the Mass of Excess Reactant: A Comprehensive Guide
Determining the mass of the excess reactant is a crucial step in many stoichiometry problems. Understanding this concept is fundamental for anyone studying chemistry, whether at a high school, undergraduate, or even graduate level. This comprehensive guide will walk you through the process step-by-step, providing clear explanations and examples to solidify your understanding. We'll cover various scenarios, from simple reactions to those involving limiting reactants and percentage yield calculations.
Understanding Stoichiometry and Limiting Reactants
Before diving into calculating the excess reactant's mass, let's establish a firm grasp of the underlying principles. Stoichiometry is the section of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. These relationships are governed by the balanced chemical equation, which provides the mole ratios of the substances involved.
A limiting reactant is the reactant that is completely consumed first in a chemical reaction. Once the limiting reactant is used up, the reaction stops, regardless of how much of the other reactants remain. The reactants that are not completely consumed are called excess reactants.
Steps to Calculate the Mass of Excess Reactant
Calculating the mass of the excess reactant involves several key steps:
-
Balance the Chemical Equation: This is the foundation of any stoichiometry problem. Ensure that the number of atoms of each element is the same on both the reactant and product sides of the equation.
-
Convert Grams to Moles: Use the molar mass of each reactant to convert the given masses (in grams) into moles. Remember, the molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol).
-
Determine the Limiting Reactant: Use the mole ratios from the balanced chemical equation to determine which reactant will be completely consumed first. Compare the mole ratio of each reactant to the actual moles you calculated in step 2. The reactant with the smaller ratio (moles of reactant / stoichiometric coefficient) is the limiting reactant.
-
Calculate Moles of Excess Reactant Consumed: Using the mole ratio from the balanced equation and the moles of the limiting reactant, calculate how many moles of the excess reactant reacted.
-
Calculate Moles of Excess Reactant Remaining: Subtract the moles of excess reactant consumed (from step 4) from the initial moles of the excess reactant (from step 2). This will give you the moles of excess reactant that remain after the reaction is complete.
-
Convert Moles to Grams: Finally, use the molar mass of the excess reactant to convert the remaining moles (from step 5) back into grams. This is the mass of the excess reactant that remains.
Example Problem: Reaction of Magnesium and Oxygen
Let's work through a detailed example to illustrate these steps. Consider the reaction between magnesium (Mg) and oxygen (O₂) to form magnesium oxide (MgO):
2Mg(s) + O₂(g) → 2MgO(s)
Suppose we react 24.3 grams of magnesium with 16.0 grams of oxygen. Let's find the mass of the excess reactant remaining after the reaction is complete.
Step 1: Balanced Equation
The equation is already balanced.
Step 2: Convert Grams to Moles
- Moles of Mg: (24.3 g Mg) / (24.3 g/mol Mg) = 1.00 mol Mg
- Moles of O₂: (16.0 g O₂) / (32.0 g/mol O₂) = 0.500 mol O₂
Step 3: Determine the Limiting Reactant
- Mg: (1.00 mol Mg) / (2 mol Mg) = 0.500
- O₂: (0.500 mol O₂) / (1 mol O₂) = 0.500
In this case, both reactants have the same ratio. This means that we can consider either as the limiting reactant for determining moles reacted, since both will be completely consumed. We’ll arbitrarily use Magnesium in the next step.
Step 4: Calculate Moles of Excess Reactant Consumed
Using the stoichiometry of the balanced equation, 1 mole of O₂ reacts with 2 moles of Mg. Since we used Mg as the limiting reactant, we'll use its amount to calculate how much oxygen was consumed:
(1.00 mol Mg) * (1 mol O₂ / 2 mol Mg) = 0.500 mol O₂ consumed
Step 5: Calculate Moles of Excess Reactant Remaining
Initial moles of O₂ - moles of O₂ consumed = moles of O₂ remaining
0.500 mol O₂ - 0.500 mol O₂ = 0 mol O₂ remaining
Step 6: Convert Moles to Grams
Since there are 0 moles of O₂ remaining, the mass of excess reactant remaining is 0 grams. Both reactants are fully consumed in this specific scenario.
Example Problem: Reaction with Unequal Ratios
Let's consider a scenario with a clear excess reactant. Suppose we react 10.0 grams of hydrogen gas (H₂) with 50.0 grams of oxygen gas (O₂) to produce water (H₂O):
2H₂(g) + O₂(g) → 2H₂O(l)
Step 1: Balanced Equation
The equation is already balanced.
Step 2: Convert Grams to Moles
- Moles of H₂: (10.0 g H₂) / (2.02 g/mol H₂) ≈ 4.95 mol H₂
- Moles of O₂: (50.0 g O₂) / (32.0 g/mol O₂) ≈ 1.56 mol O₂
Step 3: Determine the Limiting Reactant
- H₂: (4.95 mol H₂) / (2 mol H₂) ≈ 2.475
- O₂: (1.56 mol O₂) / (1 mol O₂) = 1.56
Oxygen is the limiting reactant in this scenario (smaller ratio).
Step 4: Calculate Moles of Excess Reactant Consumed
(1.56 mol O₂) * (2 mol H₂ / 1 mol O₂) ≈ 3.12 mol H₂ consumed
Step 5: Calculate Moles of Excess Reactant Remaining
Initial moles of H₂ - moles of H₂ consumed = moles of H₂ remaining
4.95 mol H₂ - 3.12 mol H₂ ≈ 1.83 mol H₂ remaining
Step 6: Convert Moles to Grams
(1.83 mol H₂) * (2.02 g/mol H₂) ≈ 3.70 g H₂ remaining
Therefore, approximately 3.70 grams of hydrogen gas remain as the excess reactant.
Incorporating Percentage Yield
In real-world scenarios, the actual yield of a reaction is often less than the theoretical yield predicted by stoichiometry. This difference is accounted for by the percentage yield. The percentage yield can be incorporated into the calculation of the excess reactant’s mass.
If you know the percentage yield, you would first calculate the theoretical yield (moles or grams) of the product based on the limiting reactant. Then, multiply this theoretical yield by the percentage yield to determine the actual yield. You can then back-calculate the moles of each reactant consumed and determine the remaining mass of the excess reactant.
Complex Reactions and Multiple Excess Reactants
The principles remain the same for more complex reactions with multiple reactants. You'll still follow the steps outlined above, determining the limiting reactant and then calculating the remaining mass of each excess reactant individually.
Conclusion
Calculating the mass of the excess reactant is an essential skill in stoichiometry. By systematically following the steps outlined in this guide – balancing the equation, converting to moles, identifying the limiting reactant, and converting back to grams – you can accurately determine the amount of excess reactant remaining after a chemical reaction. Remember to always double-check your calculations and ensure you understand the underlying concepts. Mastering this skill will strengthen your understanding of chemical reactions and their quantitative aspects, crucial for success in chemistry studies.
Latest Posts
Latest Posts
-
How Many Electrons Are In O2
Mar 23, 2025
-
What Is 60 In Fraction Form
Mar 23, 2025
-
63 Is 90 Of What Number
Mar 23, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 23, 2025
-
What Parts Of Atoms Are Involved In Chemical Reactions
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Mass Of Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
