How Many Valence Electrons In Zn
listenit
Mar 26, 2025 · 5 min read
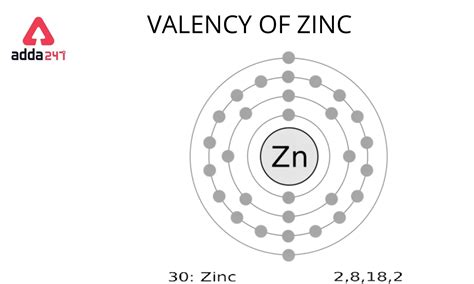
Table of Contents
How Many Valence Electrons Does Zinc (Zn) Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Zinc, a ubiquitous element crucial in numerous biological processes and industrial applications, often sparks curiosity about its electronic structure. Understanding its valence electrons is key to comprehending its chemical reactivity and the diverse roles it plays. This comprehensive guide delves into the intricacies of zinc's electronic configuration, explaining precisely how many valence electrons it possesses and why this number is so significant. We'll explore its position in the periodic table, its electron configuration, and the implications of its valence electrons for its chemical bonding and properties.
Understanding Valence Electrons: The Key to Chemical Behavior
Before focusing specifically on zinc, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the electrons most directly involved in chemical bonding and determine an element's reactivity and the types of chemical bonds it can form. The number of valence electrons dictates the element's combining capacity, determining the number of bonds it can form with other atoms.
Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas. This is achieved by either gaining, losing, or sharing electrons to fill their outermost shell, a principle known as the octet rule (although exceptions exist, particularly with elements beyond the second period). The pursuit of this stability drives chemical reactions.
Zinc's Place in the Periodic Table: Unveiling its Electronic Structure
Zinc (Zn) is a transition metal located in group 12 and period 4 of the periodic table. Its atomic number is 30, meaning it has 30 protons and, in a neutral atom, 30 electrons. Understanding its position in the periodic table helps us predict its electronic configuration. Transition metals are known for their variable oxidation states, which are directly linked to their valence electrons.
Determining Zinc's Electronic Configuration
To determine the number of valence electrons in zinc, we need to examine its electronic configuration. This describes how electrons are distributed among the various energy levels and subshells within the atom. Using the Aufbau principle (filling orbitals in order of increasing energy) and Hund's rule (maximizing unpaired electrons in degenerate orbitals), we arrive at zinc's electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰
This configuration shows a detailed distribution of electrons across various shells and subshells. The superscripts indicate the number of electrons in each subshell.
How Many Valence Electrons Does Zinc Have? The Answer Revealed
While the 4s and 3d orbitals are relatively close in energy, the 4s electrons are considered the outermost electrons and therefore the valence electrons in zinc. This is because 4s electrons are higher in energy, on average, than 3d electrons, and are thus more easily involved in chemical bonding. The filled 3d subshell is considered to be a core electron configuration.
Therefore, zinc (Zn) has 2 valence electrons.
The Significance of Zinc's Two Valence Electrons
The presence of only two valence electrons significantly influences zinc's chemical behavior. It typically loses these two electrons to achieve a stable +2 oxidation state, forming Zn²⁺ ions. This tendency explains why zinc readily forms ionic compounds with nonmetals and participates in various coordination complexes.
Implications for Chemical Bonding:
- Ionic Bonding: The loss of two valence electrons results in strong ionic bonds with electronegative elements such as oxygen, chlorine, and sulfur, forming compounds like ZnO (zinc oxide), ZnCl₂ (zinc chloride), and ZnS (zinc sulfide).
- Metallic Bonding: Zinc's metallic character arises from the delocalized nature of its valence electrons. These electrons contribute to the strong metallic bonding within the zinc metal lattice, giving it its characteristic properties, like high electrical and thermal conductivity, malleability, and ductility.
- Coordination Complexes: The Zn²⁺ ion can act as a Lewis acid (electron acceptor), forming coordination complexes with various ligands (molecules or ions that donate electron pairs). These complexes play critical roles in various biological processes.
Zinc's Role in Biology and Industry:
Zinc's unique electronic configuration and resulting chemical properties are crucial for its widespread applications:
- Biological Roles: Zinc is an essential trace element in human biology. It plays vital roles in various enzymes, acting as a catalytic center or structural component. It's involved in DNA synthesis, immune function, wound healing, and many other essential biological processes.
- Industrial Applications: Zinc's corrosion resistance makes it a vital component in galvanization, protecting iron and steel from rust. It's also used in alloys (brass, bronze), batteries, and various other industrial applications.
Beyond the Basics: Exploring Exceptions and Nuances
While the +2 oxidation state is the most common for zinc, there's ongoing research exploring the possibility of higher oxidation states under specific conditions. The energy difference between the 3d and 4s orbitals is relatively small, and under high pressures or with specific ligands, it is conceivable, though rare, for the d-electrons to participate in chemical bonding. However, these are highly specialized scenarios and, for the vast majority of cases, zinc remains a +2 ion.
Conclusion: Understanding Zinc's Valence Electrons
In conclusion, zinc (Zn) possesses two valence electrons, a feature that profoundly influences its chemical reactivity and its diverse roles in biological systems and industrial applications. Understanding its electronic configuration and the significance of its valence electrons allows us to comprehend its participation in chemical bonding, its prevalence in numerous compounds and complexes, and its crucial roles in both biological and industrial contexts. The seemingly simple number '2' holds the key to unlocking the fascinating chemistry of this essential element. This detailed exploration underscores the importance of linking atomic structure to macroscopic properties, a fundamental principle in chemistry.
Latest Posts
Latest Posts
-
68 Grams Is How Many Ounces
Mar 29, 2025
-
What Is The Square Root Of Negative 4
Mar 29, 2025
-
2002 Ap Chem Frq Form B
Mar 29, 2025
-
Where Is The Majority Of Earths Freshwater Located
Mar 29, 2025
-
What Kinds Of Elements Form Covalent Bonds
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Zn . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
