What Kinds Of Elements Form Covalent Bonds
listenit
Mar 29, 2025 · 5 min read
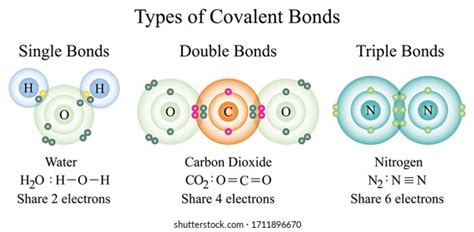
Table of Contents
What Kinds of Elements Form Covalent Bonds?
Covalent bonds are a fundamental concept in chemistry, representing the strong attractive force that holds atoms together in molecules. Understanding which elements readily form these bonds is crucial to grasping the structure and properties of a vast array of compounds, from simple diatomic gases to complex biological macromolecules. This article delves into the intricacies of covalent bonding, exploring the types of elements involved, the factors influencing bond formation, and the characteristics of resulting molecules.
The Nature of Covalent Bonds
Before diving into the specifics of element types, let's briefly review the nature of a covalent bond. Unlike ionic bonds, which involve the transfer of electrons from one atom to another, covalent bonds arise from the sharing of electrons between atoms. This sharing creates a stable, lower-energy state for the participating atoms, as it allows each atom to effectively achieve a full outer electron shell (octet rule, though there are exceptions). The shared electrons are attracted to the positively charged nuclei of both atoms, holding them together.
The strength of a covalent bond depends on several factors, including the electronegativity difference between the atoms involved, the number of shared electron pairs (single, double, or triple bonds), and the distance between the atomic nuclei.
Elements That Commonly Form Covalent Bonds
Covalent bonds are most commonly formed between nonmetal atoms. Nonmetals are elements located on the right side of the periodic table. They have high electronegativities, meaning they strongly attract electrons. This strong attraction for electrons prevents them from readily losing electrons to form ions, as happens in ionic bonding. Instead, they share electrons to achieve a stable electron configuration.
Let's examine some specific groups of nonmetals known for their propensity to form covalent bonds:
1. Halogens (Group 17):
Halogens, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), are highly reactive nonmetals. They have seven valence electrons and readily form covalent bonds to gain one more electron and achieve a stable octet. They often form diatomic molecules (e.g., F₂, Cl₂, Br₂, I₂) where two halogen atoms share a single pair of electrons. Halogens also readily form covalent compounds with other nonmetals.
Examples: Hydrogen fluoride (HF), hydrogen chloride (HCl), carbon tetrachloride (CCl₄), iodine monochloride (ICl).
2. Chalcogens (Group 16):
Chalcogens, such as oxygen (O), sulfur (S), selenium (Se), and tellurium (Te), possess six valence electrons. To achieve a stable octet, they commonly form two covalent bonds, sharing two pairs of electrons (double bond) or forming two single bonds. Oxygen is a particularly important element in covalent bonding, forming the backbone of countless organic and inorganic molecules.
Examples: Water (H₂O), carbon dioxide (CO₂), sulfur dioxide (SO₂), hydrogen sulfide (H₂S).
3. Pnictogens (Group 15):
Pnictogens like nitrogen (N), phosphorus (P), arsenic (As), and antimony (Sb) have five valence electrons. They typically form three covalent bonds to complete their octet, although sometimes they can form multiple bonds. Nitrogen is exceptionally noteworthy, forming a triple bond in diatomic nitrogen (N₂) - one of the strongest covalent bonds known.
Examples: Ammonia (NH₃), phosphine (PH₃), nitrogen dioxide (NO₂), nitric acid (HNO₃).
4. Carbon (Group 14):
Carbon, although on the border between metals and nonmetals, is considered a nonmetal and a cornerstone of organic chemistry. With four valence electrons, it readily forms four covalent bonds, leading to a vast array of organic compounds. Carbon's ability to form single, double, and triple bonds and to catenate (bond to other carbon atoms) contributes to the enormous diversity of organic molecules.
Examples: Methane (CH₄), ethane (C₂H₆), ethene (C₂H₄), ethyne (C₂H₂), countless organic polymers and biological molecules.
5. Hydrogen (Group 1):
While hydrogen is a unique element located in Group 1, it behaves more like a nonmetal in its bonding behavior. With one valence electron, it can form one covalent bond by sharing its electron with another atom. Hydrogen is ubiquitous in organic molecules and numerous inorganic compounds.
Examples: Water (H₂O), methane (CH₄), ammonia (NH₃), hydrogen chloride (HCl).
Factors Influencing Covalent Bond Formation
Several factors influence whether atoms will form covalent bonds and the characteristics of those bonds:
-
Electronegativity: The electronegativity of an atom represents its ability to attract electrons in a chemical bond. A large difference in electronegativity between two atoms leads to polar covalent bonds, where the shared electrons are closer to the more electronegative atom, creating partial charges. A small electronegativity difference results in nonpolar covalent bonds, with relatively equal sharing of electrons.
-
Valence Electrons: The number of valence electrons in an atom dictates how many covalent bonds it can form. Atoms tend to share electrons to achieve a stable electron configuration, usually a full outer shell (octet rule).
-
Atomic Size: Larger atoms have more diffuse electron clouds, leading to weaker covalent bonds compared to smaller atoms.
-
Bond Order: The bond order (number of shared electron pairs) influences the bond strength and length. Triple bonds are stronger and shorter than double bonds, which are stronger and shorter than single bonds.
Exceptions to the Octet Rule
While the octet rule serves as a useful guideline, some molecules exhibit exceptions. These exceptions arise from several reasons:
-
Electron-deficient molecules: Some molecules, like boron trifluoride (BF₃), have fewer than eight valence electrons around the central atom.
-
Electron-rich molecules: Certain molecules, like sulfur hexafluoride (SF₆), have more than eight valence electrons around the central atom. This is more common for elements in the third period and beyond, which have available d-orbitals for bonding.
-
Odd-electron molecules: Some molecules, like nitrogen dioxide (NO₂), have an odd number of valence electrons, making it impossible for all atoms to achieve an octet.
Conclusion
Covalent bonding is a fundamental process in chemistry, responsible for the formation of a vast range of molecules. While nonmetals are the primary players in covalent bond formation, understanding the roles of electronegativity, valence electrons, and atomic size helps explain the diversity and properties of covalently bonded compounds. Exceptions to the octet rule highlight the complexity of bonding and remind us that models are simplified representations of reality. The study of covalent bonds continues to be a dynamic and exciting field with implications across various branches of science and technology.
Latest Posts
Latest Posts
-
Gcf Of 42 126 And 210
Apr 01, 2025
-
What Are The First 5 Multiples Of 7
Apr 01, 2025
-
What Is 8 10 As A Decimal
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Kinds Of Elements Form Covalent Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
