How Many Valence Electrons In Ni
listenit
Mar 23, 2025 · 5 min read
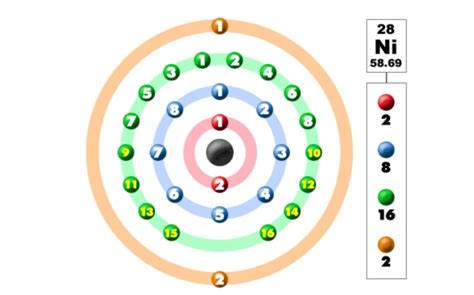
Table of Contents
How Many Valence Electrons Does Nickel Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Nickel, a silvery-white metal with the symbol Ni and atomic number 28, plays a crucial role in various industrial applications and biological processes. Understanding its chemical behavior necessitates a thorough grasp of its electronic structure, particularly the number of valence electrons it possesses. This article delves into the intricacies of nickel's electronic configuration, explaining how to determine its valence electrons and exploring the implications this has on its reactivity and bonding characteristics.
Understanding Valence Electrons: The Key to Chemical Reactivity
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's position in the periodic table and its chemical properties. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (a full outermost shell), through the gain, loss, or sharing of valence electrons.
Determining Nickel's Valence Electrons: Electronic Configuration
To determine the number of valence electrons in nickel, we must first understand its electronic configuration. This describes the arrangement of electrons in the different energy levels and sublevels within the atom. Nickel's atomic number is 28, indicating it has 28 electrons. Its electronic configuration is typically written as:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸
This notation specifies the number of electrons in each subshell. For instance, "1s²" indicates two electrons in the 1s subshell, "2s²" indicates two electrons in the 2s subshell, and so on.
Now, let's identify the valence electrons. While the outermost shell might seem to be the 4s shell with two electrons, the situation with transition metals like nickel is a bit more nuanced. The 3d and 4s subshells are very close in energy, meaning electrons can easily transition between them, particularly during chemical reactions.
Therefore, for nickel, both the 4s and 3d electrons are considered valence electrons. This means nickel typically has 10 valence electrons (2 from 4s and 8 from 3d).
Implications of Nickel's 10 Valence Electrons
The fact that nickel possesses 10 valence electrons significantly impacts its chemical behavior and bonding capabilities. Let's explore some key implications:
1. Variable Oxidation States:
Nickel's ability to readily lose electrons from both its 4s and 3d orbitals leads to variable oxidation states. Common oxidation states for nickel include +2 and +3, but it can also exhibit +1, +4, and even rarer higher oxidation states under specific conditions. This versatility in oxidation states allows nickel to participate in a wide range of chemical reactions and form diverse compounds. For example, nickel(II) oxide (NiO) has a +2 oxidation state for nickel, while nickel(III) oxide (Ni₂O₃) shows a +3 oxidation state.
2. Complex Formation:
The partially filled 3d orbitals enable nickel to form a large number of coordination complexes. Coordination complexes are formed when metal ions (like nickel) bond to ligands (molecules or ions that donate electron pairs). The ability to form these complexes is fundamental to nickel's role in various catalytic processes and its use in coordination chemistry. The geometry and stability of these complexes are influenced by factors like the number of ligands and their electronic properties.
3. Catalytic Activity:
Nickel's variable oxidation states and ability to form complexes are essential for its catalytic activity. Nickel catalysts are employed in numerous industrial processes, including:
-
Hydrogenation: The addition of hydrogen to unsaturated organic compounds. Nickel catalysts facilitate this reaction, converting unsaturated fats into saturated fats, for example, in the production of margarine.
-
Carbonylation: The reaction of carbon monoxide with other molecules. This process is crucial in the production of various chemicals, including acetic acid.
-
Hydroformylation: The addition of carbon monoxide and hydrogen to alkenes. This reaction is used to produce aldehydes, which are important building blocks for various chemicals.
4. Magnetic Properties:
Nickel's partially filled 3d orbitals contribute to its ferromagnetic properties. Ferromagnetism is a form of magnetism where a material exhibits a spontaneous and persistent magnetization even in the absence of an external magnetic field. This property makes nickel valuable in applications such as electromagnets and magnetic storage devices.
5. Alloy Formation:
Nickel readily forms alloys with other metals, significantly altering their properties. These alloys find extensive use in various industries:
-
Stainless steel: Nickel is a key component of stainless steel, enhancing its corrosion resistance and mechanical strength.
-
Nickel-based superalloys: These alloys possess exceptional high-temperature strength and are employed in gas turbine engines and aerospace applications.
-
Monel: An alloy of nickel and copper, known for its corrosion resistance and strength, used in marine environments.
Beyond the Basics: Exceptions and Refinements
While the 10 valence electron model effectively explains many of nickel's chemical behaviors, it's crucial to acknowledge certain nuances and exceptions:
-
Influence of Ligands: The actual number of electrons participating in bonding can be influenced by the nature of the ligands bound to the nickel ion. Strong-field ligands can cause electron pairing in the d orbitals, altering the effective number of valence electrons involved in bonding.
-
Excited States: Under certain conditions, such as high temperatures or strong electromagnetic fields, nickel atoms can exist in excited states where electrons are promoted to higher energy levels, temporarily changing its valence electron configuration.
-
Advanced Theoretical Models: More sophisticated theoretical models, like Density Functional Theory (DFT), provide a more precise description of electron distribution and bonding in nickel compounds, considering the complexity of electron-electron interactions.
Conclusion: A Versatile Metal with a Dynamic Electronic Structure
In summary, nickel possesses 10 valence electrons, a fact crucial to understanding its diverse chemical and physical properties. This electron count accounts for its variable oxidation states, its ability to form complexes, its catalytic activity, its magnetic properties, and its propensity for alloy formation. While a simplified model of 10 valence electrons serves as a valuable starting point, understanding the subtleties of electron configuration and ligand effects provides a deeper and more accurate picture of nickel's multifaceted behavior in the chemical world. This detailed knowledge of nickel's electronic structure underpins its widespread applications across numerous industries and research fields. Further exploration of nickel's chemistry continues to reveal new insights and innovative applications for this versatile and important metal.
Latest Posts
Latest Posts
-
Common Factors Of 30 And 42
Mar 24, 2025
-
What Does Decreased By Mean In Math
Mar 24, 2025
-
What Are The Factors For 44
Mar 24, 2025
-
Point Estimation Of The Population Mean
Mar 24, 2025
-
Lowest Common Multiple Of 36 And 45
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Ni . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
