How Many Valence Electrons In Iodine
listenit
Mar 16, 2025 · 5 min read
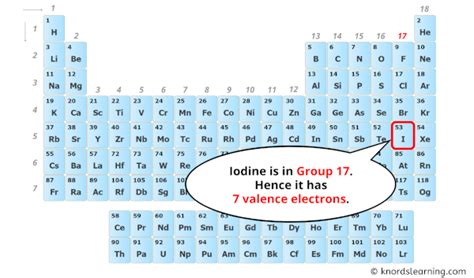
Table of Contents
How Many Valence Electrons Does Iodine Have? A Deep Dive into Iodine's Electronic Structure
Iodine, a fascinating halogen element, plays a crucial role in various biological and chemical processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and properties. This comprehensive guide delves into the intricacies of iodine's electron configuration, explaining why it possesses the number of valence electrons it does and exploring the implications of this for its chemical behavior.
Understanding Valence Electrons: The Key to Reactivity
Before we pinpoint the number of valence electrons in iodine, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the ones most involved in chemical bonding, determining an atom's reactivity and the types of bonds it can form. They dictate how an atom will interact with other atoms to form molecules and compounds. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas, by gaining, losing, or sharing valence electrons.
Iodine's Atomic Structure and Electron Configuration
Iodine (I) is a chemical element with atomic number 53. This means that a neutral iodine atom contains 53 protons and 53 electrons. To determine the number of valence electrons, we need to examine iodine's electron configuration. The electron configuration represents the arrangement of electrons in different energy levels or shells within the atom.
Iodine's electron configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵.
This notation indicates how many electrons occupy each subshell within each principal energy level. The principal energy levels (n=1, 2, 3, etc.) represent the shells, while the subshells (s, p, d, f) represent the different regions within a shell where electrons can be found.
Identifying Valence Electrons in Iodine
The outermost shell of an atom is its highest principal energy level. In iodine's electron configuration, the highest principal energy level is n=5. This level contains the 5s and 5p subshells.
Therefore, iodine's valence electrons are those located in the 5s and 5p subshells:
- 5s²: Two electrons in the 5s subshell.
- 5p⁵: Five electrons in the 5p subshell.
Adding these together, we find that iodine has a total of seven valence electrons (2 + 5 = 7).
Implications of Seven Valence Electrons: Iodine's Chemical Behavior
The presence of seven valence electrons significantly influences iodine's chemical behavior. Atoms tend to react in ways that allow them to achieve a stable, filled outermost shell, usually with eight electrons (the octet rule, although there are exceptions). Iodine, with seven valence electrons, is one electron short of a full octet. This makes it highly reactive, readily participating in chemical reactions to gain an additional electron and achieve a stable electron configuration.
This tendency leads to several important characteristics of iodine:
- High electronegativity: Iodine has a relatively high electronegativity, meaning it has a strong tendency to attract electrons towards itself in a chemical bond.
- Formation of ionic and covalent bonds: Iodine can form both ionic and covalent bonds. In ionic bonds, it readily gains an electron to become an iodide ion (I⁻), achieving a stable octet. In covalent bonds, it shares electrons with other atoms to achieve a stable configuration, often forming single bonds.
- Oxidation states: Due to its ability to gain or share electrons, iodine can exhibit various oxidation states, ranging from -1 (iodide ion) to +7.
- Formation of polyatomic ions: Iodine can participate in the formation of polyatomic ions, such as the iodate ion (IO₃⁻) and periodate ion (IO₄⁻).
Specific examples of iodine's reactivity driven by its seven valence electrons:
- Reaction with metals: Iodine readily reacts with many metals to form metal iodides. For example, the reaction of sodium (Na) with iodine (I₂) produces sodium iodide (NaI), where iodine gains an electron to become an iodide ion.
- Reaction with non-metals: Iodine also reacts with other non-metals, forming covalent compounds. A prime example is the formation of hydrogen iodide (HI), a strong acid, where iodine shares an electron with hydrogen.
- Formation of interhalogen compounds: Iodine can form compounds with other halogens (fluorine, chlorine, bromine), creating interhalogen compounds like iodine monochloride (ICl) and iodine pentafluoride (IF₅). This is a consequence of its ability to share electrons with other highly electronegative atoms.
Beyond the Octet Rule: Exceptions and Complexities
While the octet rule provides a useful framework for understanding chemical bonding, it's important to acknowledge that there are exceptions, and iodine's behavior sometimes deviates from it. For instance, in some compounds, iodine may exhibit an expanded octet, meaning it can accommodate more than eight electrons in its valence shell. This is possible because iodine has available d orbitals that can participate in bonding.
Iodine in Biology and its Importance of Valence Electrons
The seven valence electrons are crucial for iodine's biological role. Iodine is an essential element for humans and animals, primarily known for its role in the production of thyroid hormones, thyroxine (T₄) and triiodothyronine (T₃). These hormones are crucial for regulating metabolism, growth, and development. The ability of iodine to form stable bonds with carbon and oxygen within the structure of these hormones is directly related to its valence electrons.
Conclusion: The Significance of Iodine's Seven Valence Electrons
In conclusion, iodine possesses seven valence electrons, a fact that profoundly impacts its chemical properties and biological significance. Its tendency to gain an electron to complete its octet or share electrons to achieve stability underpins its reactivity and the formation of a wide array of compounds. Understanding iodine's electron configuration and the behavior of its valence electrons is crucial for comprehending its diverse roles in chemistry, biology, and various industrial applications. This knowledge lays the groundwork for further exploration of iodine's fascinating chemical and biological properties. Further research into iodine’s complex interactions and its unique behavior in various contexts offers exciting possibilities for advancement in many fields.
Latest Posts
Latest Posts
-
What Is The Gcf Of 20 And 16
Mar 16, 2025
-
What Is The Conjugate Acid Of Hco3
Mar 16, 2025
-
A Ma Or An Ma Degree
Mar 16, 2025
-
What Is 3 Square Root Of 3
Mar 16, 2025
-
5 1 2 To Improper Fraction
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Iodine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
