What Is The Conjugate Acid Of Hco3
listenit
Mar 16, 2025 · 5 min read
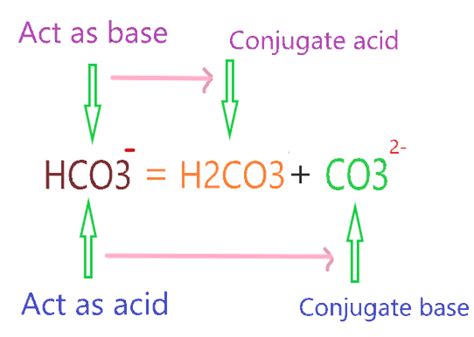
Table of Contents
What is the Conjugate Acid of HCO₃⁻? A Deep Dive into Acid-Base Chemistry
The bicarbonate ion, HCO₃⁻, plays a crucial role in various biological and chemical processes. Understanding its behavior as a Brønsted-Lowry base, and subsequently identifying its conjugate acid, is fundamental to grasping these processes. This article provides a comprehensive exploration of the conjugate acid of HCO₃⁻, delving into the concepts of acids, bases, conjugate pairs, and the equilibrium involved. We'll also examine the significance of HCO₃⁻ and its conjugate acid in different contexts.
Understanding Brønsted-Lowry Acids and Bases
Before we pinpoint the conjugate acid of HCO₃⁻, it's vital to refresh our understanding of Brønsted-Lowry acid-base theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. A crucial aspect of this theory is the concept of conjugate acid-base pairs.
When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton (H⁺).
Identifying the Conjugate Acid of HCO₃⁻
The bicarbonate ion (HCO₃⁻) acts as a Brønsted-Lowry base. This means it can accept a proton (H⁺) from an acid. To find its conjugate acid, we simply add a proton to its structure.
Adding a proton (H⁺) to HCO₃⁻ yields H₂CO₃, carbonic acid. Therefore, the conjugate acid of HCO₃⁻ is H₂CO₃.
This reaction can be represented as follows:
HCO₃⁻(aq) + H⁺(aq) ⇌ H₂CO₃(aq)
This equation shows the reversible nature of the reaction. The equilibrium between HCO₃⁻, H⁺, and H₂CO₃ is crucial in many systems, particularly in blood buffering.
The Importance of Carbonic Acid (H₂CO₃)
Carbonic acid (H₂CO₃), the conjugate acid of HCO₃⁻, is a weak diprotic acid. This means it can donate two protons. Its importance stems from its role in various biological and chemical processes:
1. Blood Buffering System: A Crucial Role
The bicarbonate buffer system is one of the most critical mechanisms maintaining the pH of human blood within a narrow, life-sustaining range (around 7.35-7.45). This system involves the equilibrium between carbonic acid (H₂CO₃), bicarbonate ions (HCO₃⁻), and hydrogen ions (H⁺):
H₂CO₃ ⇌ H⁺ + HCO₃⁻
This equilibrium acts as a buffer, resisting changes in pH. When an excess of H⁺ (acid) enters the blood, the equilibrium shifts to the left, forming more H₂CO₃. Conversely, if the blood becomes too alkaline (lack of H⁺), the equilibrium shifts to the right, releasing more H⁺.
2. Ocean Acidification: A Global Concern
The ocean absorbs a significant amount of atmospheric CO₂. This dissolved CO₂ reacts with water to form carbonic acid (H₂CO₃):
CO₂(aq) + H₂O(l) ⇌ H₂CO₃(aq)
Carbonic acid then dissociates, releasing H⁺ and HCO₃⁻ ions. This process contributes to ocean acidification, a serious environmental issue with far-reaching consequences for marine life. The increase in H⁺ ions lowers the pH of seawater, impacting the ability of marine organisms, such as corals and shellfish, to build and maintain their calcium carbonate shells and skeletons.
3. Chemical Applications: Diverse Uses
Beyond its biological roles, carbonic acid and its derivatives find applications in various chemical processes. For example, it is used in the production of certain salts and as a component in some food and beverage products.
Equilibrium and the Acid Dissociation Constant (Ka)
The equilibrium between HCO₃⁻ and its conjugate acid, H₂CO₃, is governed by its acid dissociation constant (Ka). The Ka value reflects the strength of an acid; a smaller Ka indicates a weaker acid, and a larger Ka indicates a stronger acid. Since H₂CO₃ is a weak acid, its Ka value is relatively small.
Understanding the Ka value helps predict the relative concentrations of H₂CO₃ and HCO₃⁻ at equilibrium under various conditions. This knowledge is essential in various fields, including environmental science, biochemistry, and chemical engineering.
Comparing HCO₃⁻ and H₂CO₃: Acidic vs. Basic Properties
HCO₃⁻ and H₂CO₃ exhibit different behaviors depending on the context:
-
HCO₃⁻ (Bicarbonate ion): Can act as both an acid and a base (amphiprotic). It can donate a proton to become CO₃²⁻ (carbonate ion), or accept a proton to become H₂CO₃ (carbonic acid).
-
H₂CO₃ (Carbonic acid): Acts primarily as an acid. It can donate a proton to become HCO₃⁻.
Their dual nature emphasizes the importance of understanding the specific conditions (pH, presence of other acids or bases) influencing their behavior.
Conjugate Acid-Base Pairs and Their Significance in Chemistry
The concept of conjugate acid-base pairs is crucial for understanding acid-base reactions and equilibria. Knowing the conjugate acid of a base or the conjugate base of an acid helps us predict reaction outcomes and understand the role of different species in various systems. This concept extends far beyond just the HCO₃⁻/H₂CO₃ pair and applies to numerous other acid-base systems throughout chemistry.
Practical Applications and Further Exploration
The knowledge of the conjugate acid of bicarbonate has practical implications across multiple disciplines. From understanding blood pH regulation in medicine to mitigating the effects of ocean acidification in environmental science, this concept is fundamental. Further exploration can involve studying the kinetics of the reactions involving HCO₃⁻ and H₂CO₃, analyzing the effects of different factors on the equilibrium, and investigating other relevant chemical systems.
In conclusion, the conjugate acid of HCO₃⁻ is H₂CO₃, carbonic acid. The significance of this relationship extends far beyond a simple acid-base reaction. Understanding this conjugate pair and their interplay in various equilibrium systems is essential for comprehending numerous chemical and biological processes. The reversible nature of the reaction between HCO₃⁻ and H₂CO₃ highlights their dynamic roles in maintaining homeostasis, influencing environmental conditions, and shaping chemical reactions. Continued investigation into these crucial components will remain critical in advancing scientific understanding and addressing crucial environmental challenges.
Latest Posts
Latest Posts
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
-
What Is 10 To The Power Of 7
Mar 17, 2025
-
Lowest Common Multiple Of 4 And 10
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Hco3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
