How Many Valence Electrons In Be
listenit
Mar 23, 2025 · 5 min read
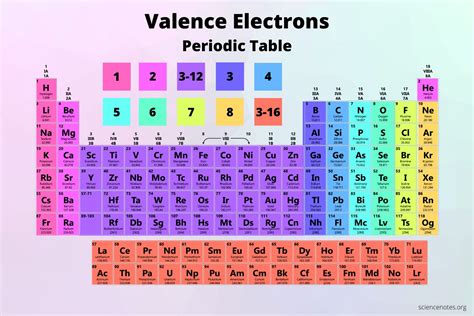
Table of Contents
How Many Valence Electrons Does Beryllium (Be) Have? A Deep Dive into Atomic Structure
Understanding the number of valence electrons in an element is crucial for comprehending its chemical behavior and bonding properties. This article delves deep into the case of beryllium (Be), exploring its atomic structure, electron configuration, and the significance of its valence electrons in determining its reactivity and the compounds it forms. We'll go beyond a simple answer, providing a comprehensive explanation accessible to both beginners and those seeking a more advanced understanding.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before focusing on beryllium, let's establish a solid foundation. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary participants in chemical bonding, dictating how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons directly influences an element's reactivity, oxidation state, and the types of bonds it can form (ionic, covalent, metallic).
Beryllium's Position on the Periodic Table: A Clue to its Valence Electrons
Beryllium (Be) is an alkaline earth metal, located in Group 2 (also known as IIA) of the periodic table. The periodic table's organization is not arbitrary; it reflects the underlying structure of atoms. Elements within the same group share similar valence electron configurations, leading to similar chemical properties. This fundamental principle provides a quick way to determine the number of valence electrons for many elements.
The key takeaway here is that elements in Group 2 of the periodic table typically have two valence electrons.
Beryllium's Electron Configuration: A Detailed Look
To confirm the number of valence electrons in beryllium, we can examine its electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Beryllium's atomic number is 4, meaning it has four protons and, in a neutral atom, four electrons.
The electron configuration of beryllium is 1s²2s². This notation tells us:
- 1s²: Two electrons occupy the 1s orbital (the lowest energy level).
- 2s²: Two electrons occupy the 2s orbital (the next higher energy level).
The outermost shell in beryllium is the second shell (n=2), and it contains two electrons. These two electrons in the 2s orbital are the valence electrons of beryllium.
Why Only Two Valence Electrons Matter in Chemical Reactions
While beryllium has four electrons in total, only the two in the outermost shell actively participate in chemical bonding. The inner shell electrons (the two in the 1s orbital) are tightly bound to the nucleus and are shielded from interaction with other atoms. They are effectively inert in chemical reactions. This shielding effect is a crucial concept in understanding atomic behavior.
Beryllium's Chemical Behavior: A Consequence of its Two Valence Electrons
Beryllium's two valence electrons explain many of its characteristic chemical properties:
- Reactivity: Beryllium is relatively reactive, although less so than other alkaline earth metals. Its two valence electrons are relatively easily lost to achieve a stable electron configuration (like helium, with a full outer shell). This loss of electrons results in the formation of Be²⁺ ions.
- Oxidation State: Beryllium typically exhibits an oxidation state of +2, reflecting the loss of its two valence electrons during chemical reactions.
- Bonding: Beryllium predominantly forms ionic bonds with highly electronegative elements like oxygen and halogens. It can also form covalent bonds, although this is less common.
Examples of Beryllium Compounds and their Formation
Let's consider some examples of beryllium compounds and how the two valence electrons play a crucial role in their formation:
-
Beryllium oxide (BeO): Beryllium readily reacts with oxygen to form beryllium oxide. Each beryllium atom loses its two valence electrons to two oxygen atoms, resulting in the formation of Be²⁺ and O²⁻ ions. These ions are then held together by strong electrostatic forces, forming an ionic crystal structure.
-
Beryllium chloride (BeCl₂): Similarly, beryllium reacts with chlorine to form beryllium chloride. Each beryllium atom loses its two valence electrons to two chlorine atoms, forming Be²⁺ and Cl⁻ ions. Again, the electrostatic attraction between these oppositely charged ions results in an ionic compound.
-
Organoberyllium compounds: Beryllium can also form covalent bonds, particularly with carbon-containing groups, forming organoberyllium compounds. In these compounds, beryllium shares its valence electrons with carbon atoms to form covalent bonds. However, even in covalent bonding, the tendency of beryllium to lose its two electrons is still influential in its chemical behavior.
Beyond the Basics: Exceptions and Nuances
While the general rule for Group 2 elements having two valence electrons holds true for beryllium in most cases, there are some nuances and exceptions to consider in more advanced chemical scenarios. The simple model we've presented is a great starting point, but a deeper dive into quantum mechanics and molecular orbital theory reveals more complex interactions, especially in unusual chemical environments or excited states.
The Importance of Valence Electrons in Various Fields
Understanding valence electrons isn't just an academic exercise; it has significant implications across various scientific and technological fields:
- Materials Science: The knowledge of valence electrons is fundamental in designing and developing new materials with specific properties. For example, understanding beryllium's valence electrons helps in designing alloys with desired strength, lightness, and other characteristics.
- Chemistry: Valence electrons are essential for predicting chemical reactions, understanding molecular structures, and designing new compounds with specific functions.
- Nanotechnology: The manipulation of individual atoms and molecules relies heavily on understanding their valence electrons and how they interact.
Conclusion: Beryllium's Two Valence Electrons – A Foundation for Understanding
In conclusion, beryllium (Be) possesses two valence electrons. This seemingly simple fact underpins its chemical behavior, reactivity, and the types of compounds it forms. By understanding the role of valence electrons, we gain a deeper appreciation for the periodic table's organization and the fundamental principles governing chemical interactions. This knowledge is not only important for understanding basic chemistry but is also crucial for advancements in materials science, nanotechnology, and other scientific fields. Further exploration into the intricacies of atomic structure and bonding will provide an even richer understanding of the fascinating world of chemistry and materials science.
Latest Posts
Latest Posts
-
Is Sohcahtoa Only For Right Triangles
Mar 24, 2025
-
Organisms That Cannot Produce Their Own Food
Mar 24, 2025
-
1 2 3g 2 G 2
Mar 24, 2025
-
Which Em Wave Has The Longest Wavelength
Mar 24, 2025
-
Which Organelles Are Involved In Energy Conversion
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Be . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
