How Many Valence Electrons In Ar
listenit
Mar 26, 2025 · 5 min read
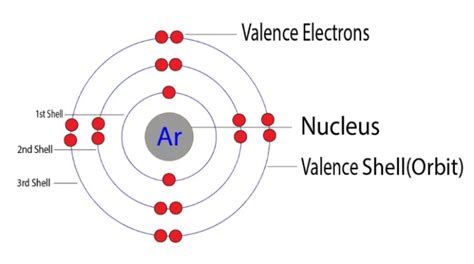
Table of Contents
How Many Valence Electrons Does Argon Have? A Deep Dive into Atomic Structure
Argon (Ar), a noble gas, holds a special place in the periodic table. Its unique atomic structure, particularly its number of valence electrons, dictates its chemical behavior and properties. Understanding this structure is crucial for comprehending its role in various applications, from lighting to industrial processes. This article will delve deep into the atomic structure of argon, explaining why it possesses the number of valence electrons it does, and the implications of this electron configuration.
Understanding Valence Electrons
Before diving into argon's specifics, let's define what valence electrons are. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most crucial in determining an atom's chemical behavior. They participate in chemical bonding, dictating whether an atom will readily react with other atoms or remain inert. The number of valence electrons determines an element's group (column) in the periodic table, reflecting its chemical properties.
Electron Shells and Subshells
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons occupying various energy levels known as shells or orbitals. These shells are arranged in increasing energy levels, with electrons filling the lower energy levels first. Each shell can only accommodate a specific number of electrons. The first shell (n=1) can hold a maximum of two electrons, the second shell (n=2) can hold up to eight electrons, and so on.
Within each shell, there are subshells, designated as s, p, d, and f, each capable of holding a specific number of electrons. The 's' subshell holds a maximum of two electrons, the 'p' subshell holds six electrons, the 'd' subshell holds ten, and the 'f' subshell holds fourteen.
Argon's Electronic Configuration: The Key to its Valence Electrons
Argon's atomic number is 18, meaning it possesses 18 protons and 18 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electronic configuration, which describes how these electrons are distributed among the various shells and subshells.
Argon's electronic configuration is 1s²2s²2p⁶3s²3p⁶. Let's break this down:
- 1s²: Two electrons occupy the first shell's 's' subshell.
- 2s²: Two electrons occupy the second shell's 's' subshell.
- 2p⁶: Six electrons occupy the second shell's 'p' subshell.
- 3s²: Two electrons occupy the third shell's 's' subshell.
- 3p⁶: Six electrons occupy the third shell's 'p' subshell.
This configuration reveals that argon's outermost shell (the third shell) is completely filled with eight electrons (two in the 3s subshell and six in the 3p subshell).
The Significance of a Full Valence Shell: Argon's Inertness
Therefore, Argon has 8 valence electrons. This full outermost shell is the key to understanding argon's chemical inertness. Atoms are most stable when their outermost electron shell is completely filled. This stable configuration minimizes their energy and reduces their reactivity. Argon, with its complete octet (eight valence electrons), exhibits very low reactivity. It rarely forms chemical bonds with other atoms because it doesn't need to gain, lose, or share electrons to achieve a more stable configuration.
Noble Gases and their Valence Electrons
Argon belongs to the noble gases, also known as inert gases. All noble gases are characterized by having a full valence shell. Helium (He) with two valence electrons (1s²) and Neon (Ne) with eight valence electrons (1s²2s²2p⁶) are other examples. This full valence shell contributes significantly to their chemical inactivity and makes them unique among the elements.
Argon's Applications: Leveraging its Inertness
Argon's inertness is exploited in various applications:
1. Welding and Metallurgy:
Argon's non-reactive nature makes it ideal as a shielding gas in welding processes. It prevents the molten metal from reacting with oxygen or nitrogen in the air, ensuring a cleaner and stronger weld. This is particularly important in welding reactive metals like aluminum and titanium.
2. Lighting:
Argon is used in incandescent and fluorescent lights. Its presence helps to prevent the rapid oxidation of the filament in incandescent bulbs and enhances the efficiency of fluorescent tubes.
3. Industrial Processes:
Argon finds applications in various industrial processes, including:
- Chemical synthesis: Its inertness makes it suitable as a protective atmosphere during certain chemical reactions.
- Food packaging: Argon is used to create a modified atmosphere in food packaging, extending the shelf life of products.
- Semiconductor manufacturing: Argon plasma is used in etching and deposition processes in semiconductor manufacturing.
4. Medical Applications:
Argon laser treatment is used in various medical procedures, including ophthalmology and dermatology.
Conclusion: Understanding Argon's Valence Electrons is Key
The fact that argon possesses eight valence electrons is fundamental to understanding its chemical properties and its wide range of applications. Its full valence shell leads to its exceptional stability and inertness, making it a valuable resource in various industries and technological processes. From welding to medical applications, Argon's unique atomic structure plays a critical role in shaping its utility and importance in modern technology and society. Its unreactive nature makes it a vital tool, preventing unwanted chemical reactions and ensuring high-quality outcomes in diverse fields. Further research into the behavior of noble gases and their valence electrons continues to open up new avenues for technological advancement and scientific discovery. The simple eight-electron count of argon's valence shell unlocks a world of possibilities.
Latest Posts
Latest Posts
-
How Much Is 6 Quarts Of Water
Mar 29, 2025
-
X 2 16 X 4 X 4
Mar 29, 2025
-
Write A Linear Function F With The Given Values
Mar 29, 2025
-
Is 35 Prime Or Composite Number
Mar 29, 2025
-
How To Find X Intercept In Vertex Form
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Ar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
