How Many Valence Electrons Does The Alkaline Earth Metals Have
listenit
Apr 01, 2025 · 6 min read
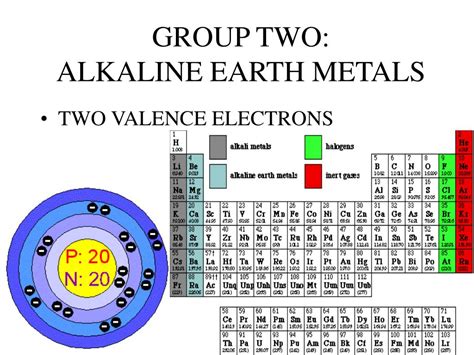
Table of Contents
How Many Valence Electrons Do Alkaline Earth Metals Have? A Deep Dive into Group 2 Elements
The alkaline earth metals, a vibrant group residing in the second column of the periodic table, are characterized by their unique chemical properties, largely governed by their electron configuration. Understanding their valence electron count is key to comprehending their reactivity and behavior. This article delves deep into the electronic structure of alkaline earth metals, explaining why they consistently possess two valence electrons, exploring the consequences of this configuration, and examining its implications in various chemical reactions and applications.
Understanding Valence Electrons: The Key to Reactivity
Before diving into the specifics of alkaline earth metals, let's establish a foundational understanding of valence electrons. Valence electrons are the outermost electrons in an atom, residing in the highest energy level. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's position on the periodic table and its chemical behavior. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling a noble gas (a group 18 element with a full outer electron shell). This drive towards stability is the driving force behind most chemical reactions.
The Significance of the Octet Rule
The octet rule, a cornerstone of chemical bonding theory, states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell. This stable configuration mimics the electron arrangement of noble gases, which are exceptionally unreactive due to their full valence shells. While not universally applicable, the octet rule provides a valuable framework for understanding the behavior of many elements, including the alkaline earth metals.
Alkaline Earth Metals: A Closer Look at Group 2
The alkaline earth metals comprise the elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements share several common characteristics, primarily stemming from their similar electronic structures. The defining feature of this group is the presence of two valence electrons in their outermost energy level (s-orbital). This consistent configuration accounts for their shared chemical properties and reactivity.
Electronic Configuration and Valence Electrons
Let's examine the electronic configurations of the first few alkaline earth metals to illustrate the consistent presence of two valence electrons:
- Beryllium (Be): 1s² 2s² (two valence electrons in the 2s orbital)
- Magnesium (Mg): 1s² 2s² 2p⁶ 3s² (two valence electrons in the 3s orbital)
- Calcium (Ca): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² (two valence electrons in the 4s orbital)
Notice the pattern: regardless of the number of inner electron shells, each alkaline earth metal consistently possesses two electrons in its outermost s-orbital. These two electrons are readily available for participation in chemical bonding.
Chemical Reactivity: The Consequences of Two Valence Electrons
The presence of two valence electrons directly influences the chemical behavior of alkaline earth metals. These metals are relatively reactive, although less so than the alkali metals (Group 1), which have only one valence electron. This reactivity is driven by their tendency to lose these two valence electrons to achieve a stable, noble gas-like configuration.
Ionization and Formation of 2+ Ions
Alkaline earth metals readily lose their two valence electrons, forming divalent cations with a 2+ charge. This ionization process requires energy, but the energy released upon formation of stable ionic bonds often outweighs this ionization energy. The resulting 2+ ions are relatively stable, contributing to the formation of numerous ionic compounds.
Examples of Chemical Reactions
The reactivity of alkaline earth metals is evident in their reactions with various substances:
-
Reaction with Oxygen: Alkaline earth metals readily react with oxygen in the air to form metal oxides. For instance, magnesium burns brightly in air to produce magnesium oxide (MgO).
-
Reaction with Water: The reactivity with water increases down the group. Beryllium does not react readily, while calcium, strontium, and barium react vigorously, producing metal hydroxides and hydrogen gas.
-
Reaction with Acids: Alkaline earth metals react with acids, producing hydrogen gas and the corresponding metal salt. This reaction is often vigorous and exothermic.
-
Formation of Ionic Compounds: Due to their tendency to form 2+ ions, alkaline earth metals readily form ionic compounds with nonmetals, such as halides (chlorides, bromides, iodides), sulfates, and carbonates. These compounds have numerous applications in various industries.
Applications of Alkaline Earth Metals and Their Compounds
The unique properties of alkaline earth metals and their compounds lead to a wide range of applications:
-
Magnesium: Used extensively in lightweight alloys for aerospace and automotive industries due to its strength and low density. Also used in flash photography and pyrotechnics due to its bright burning characteristics. Magnesium compounds find applications in medicine as laxatives and antacids.
-
Calcium: An essential element in human biology, playing a vital role in bone structure and muscle function. Calcium carbonate (CaCO₃) is used in construction materials (cement, limestone), as a filler in paper production, and as an antacid. Calcium sulfate (CaSO₄) is used in plaster and drywall.
-
Strontium: Used in fireworks to produce a red color. Strontium carbonate is employed in the production of ferrite magnets.
-
Barium: Barium sulfate (BaSO₄) is used as a contrast agent in medical imaging (X-rays) due to its high opacity to X-rays. Barium compounds also find applications in the production of glass and ceramics.
-
Radium: A radioactive element with limited applications due to its radioactivity, although historically it was used in luminous paints.
Conclusion: The Importance of Valence Electrons in Defining Chemical Behavior
The consistent presence of two valence electrons in alkaline earth metals is a defining characteristic that dictates their chemical behavior and reactivity. Their tendency to lose these two electrons to form 2+ ions drives their participation in various chemical reactions and results in the formation of numerous ionic compounds with diverse applications. Understanding the electronic configuration and the role of valence electrons is crucial for comprehending the properties and applications of this important group of elements. From lightweight alloys to essential biological roles, the alkaline earth metals demonstrate the profound impact of electronic structure on the macroscopic world around us. The consistent two valence electrons act as a fundamental building block shaping the properties and uses of these metals, a testament to the elegance and predictive power of the periodic table. Further research and development continue to uncover new applications for these elements, underscoring their lasting significance in various scientific and technological fields. Continued study of their unique electronic structure promises to unveil even more about their potential uses and contributions to future innovations.
Latest Posts
Latest Posts
-
What Is The Muscle That Subdivides The Ventral Body Cavity
Apr 02, 2025
-
How Do I Graph X 1
Apr 02, 2025
-
Lowest Common Multiple Of 32 And 48
Apr 02, 2025
-
What Is The Greatest Common Factor Of 8 And 24
Apr 02, 2025
-
A Solution In Which The Solvent Is Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does The Alkaline Earth Metals Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
