How Many Valence Electrons Does Sulfer Have
listenit
Mar 23, 2025 · 6 min read
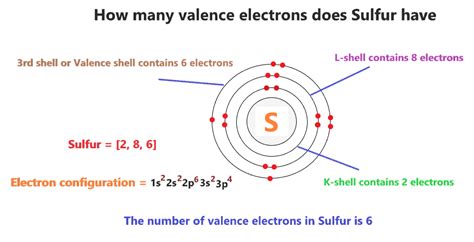
Table of Contents
- How Many Valence Electrons Does Sulfer Have
- Table of Contents
- How Many Valence Electrons Does Sulfur Have? A Deep Dive into Sulfur's Electronic Structure
- Understanding Valence Electrons: The Key to Reactivity
- How to Determine Valence Electrons
- Delving into Sulfur's Electronic Structure
- Sulfur's Position on the Periodic Table
- Sulfur's Electron Configuration
- Visualizing Sulfur's Valence Electrons with Lewis Dot Structures
- The Implications of Sulfur's Six Valence Electrons
- Sulfur's Diverse Chemical Interactions
- Sulfur's Significance in Biology and Industry
- Addressing Common Misconceptions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How Many Valence Electrons Does Sulfur Have? A Deep Dive into Sulfur's Electronic Structure
Sulfur, a vibrant yellow nonmetal, plays a crucial role in various biological and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article delves deep into the electronic configuration of sulfur, explaining how to determine its valence electrons and exploring the implications of this characteristic in its diverse chemical interactions.
Understanding Valence Electrons: The Key to Reactivity
Before focusing on sulfur, let's establish a firm understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they are the ones involved in chemical bonding. They determine an atom's reactivity, its ability to form bonds with other atoms, and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons directly influences an element's position in the periodic table and its chemical properties.
How to Determine Valence Electrons
There are several ways to determine the number of valence electrons an atom possesses:
-
Using the Periodic Table: The most straightforward method is to use the periodic table's group number (vertical columns). For main group elements (groups 1-18), the group number generally corresponds to the number of valence electrons. However, there are exceptions, especially for transition metals.
-
Electron Configuration: Analyzing the electron configuration of an atom reveals the distribution of electrons across different energy levels (shells and subshells). The electrons in the outermost shell are the valence electrons.
-
Lewis Dot Structures: Lewis dot structures are visual representations of valence electrons. Each dot represents a valence electron, placed around the element's symbol.
Delving into Sulfur's Electronic Structure
Sulfur (S) has an atomic number of 16, meaning it possesses 16 protons and, in its neutral state, 16 electrons. To determine the number of valence electrons in sulfur, we'll employ both the periodic table method and the electron configuration approach.
Sulfur's Position on the Periodic Table
Sulfur is located in Group 16 (or VIA) of the periodic table. Elements in Group 16 typically have six valence electrons.
Sulfur's Electron Configuration
The electron configuration of sulfur is 1s²2s²2p⁶3s²3p⁴. Let's break this down:
- 1s²: Two electrons in the first energy level (shell).
- 2s²: Two electrons in the second energy level.
- 2p⁶: Six electrons in the second energy level's p subshell.
- 3s²: Two electrons in the third energy level.
- 3p⁴: Four electrons in the third energy level's p subshell.
The outermost energy level for sulfur is the third energy level (n=3). This level contains a total of six electrons (3s² + 3p⁴). Therefore, sulfur has six valence electrons.
Visualizing Sulfur's Valence Electrons with Lewis Dot Structures
A Lewis dot structure for sulfur would be represented as:
..
: S :
..
The six dots surrounding the 'S' symbol represent sulfur's six valence electrons.
The Implications of Sulfur's Six Valence Electrons
The presence of six valence electrons profoundly impacts sulfur's chemical behavior. To achieve a stable octet (eight electrons in its outermost shell), sulfur commonly forms two covalent bonds, sharing two of its electrons to complete its octet. However, it is important to note that sulfur can also form multiple bonds and even expand its octet.
Sulfur's Diverse Chemical Interactions
Sulfur's six valence electrons enable it to participate in a wide array of chemical reactions and form numerous compounds. Some key aspects of sulfur's chemistry due to its six valence electrons include:
-
Covalent Bonding: Sulfur frequently forms covalent bonds with other nonmetals, sharing electron pairs to achieve a stable octet. Examples include hydrogen sulfide (H₂S), sulfur dioxide (SO₂), and sulfur trioxide (SO₃).
-
Ionic Bonding: While less common than covalent bonding, sulfur can form ionic bonds with highly electropositive metals, transferring electrons to become a sulfide ion (S²⁻). This occurs in compounds like sodium sulfide (Na₂S) and iron sulfide (FeS).
-
Multiple Bonds: Sulfur exhibits variable oxidation states, allowing it to form double and even triple bonds. For instance, in sulfur dioxide (SO₂), sulfur forms a double bond with one oxygen atom and a single bond with another.
-
Expanded Octet: In certain molecules, sulfur can exceed the octet rule, accommodating more than eight electrons in its valence shell. This phenomenon is observed in molecules such as sulfur hexafluoride (SF₆).
-
Allotropes: Sulfur's ability to form different allotropes (different structural forms of the same element) is partly due to its six valence electrons. These allotropes include rhombic sulfur, monoclinic sulfur, and plastic sulfur. Each allotrope exhibits different physical and chemical properties due to variations in its bonding and structure.
Sulfur's Significance in Biology and Industry
Sulfur’s unique chemical properties, dictated by its six valence electrons, contribute to its importance in various biological and industrial processes:
-
Biological Roles: Sulfur is an essential component of many biomolecules, including proteins (cysteine and methionine amino acids), vitamins (thiamine and biotin), and coenzymes. Its ability to form disulfide bonds plays a vital role in protein structure and function.
-
Industrial Applications: Sulfur is extensively used in the production of sulfuric acid (H₂SO₄), a cornerstone chemical in numerous industrial processes, including fertilizer production, metal refining, and petroleum refining. Sulfuric acid's high reactivity and versatile nature are directly related to sulfur's electronic structure and bonding capabilities. Furthermore, sulfur is also employed in the vulcanization of rubber, providing strength and durability.
Addressing Common Misconceptions
Understanding sulfur's valence electrons can help dispel some common misconceptions:
-
Confusing Valence Electrons with Total Electrons: It’s crucial to distinguish between the total number of electrons in an atom and the number of valence electrons. While sulfur has 16 electrons overall, only six are valence electrons.
-
Assuming a Fixed Number of Bonds: Although sulfur often forms two bonds to complete its octet, it's important to remember its ability to form multiple bonds and expand its octet, leading to diverse bonding arrangements.
-
Oversimplifying Chemical Behavior: The number of valence electrons is a starting point for understanding an element's reactivity. However, other factors like electronegativity, atomic size, and the presence of other atoms in a molecule also play crucial roles in determining the specific chemical behavior.
Conclusion
Sulfur, with its six valence electrons, exemplifies how an element's electronic structure dictates its chemical behavior and reactivity. Its capacity for covalent and ionic bonding, multiple bonding, and octet expansion contributes to its diverse roles in biological systems and industrial processes. A solid understanding of valence electrons is essential for predicting sulfur's chemical interactions and appreciating its significant contributions to both the natural world and human technological advancements. The number six, therefore, is not just a numerical value but a key to unlocking the fascinating chemistry of this versatile element. Further exploration into sulfur's chemistry unveils even more intricate details, reinforcing the pivotal role of valence electrons in shaping the properties and behavior of elements.
Latest Posts
Latest Posts
-
What Is The Opposite Of Condensation
Mar 26, 2025
-
9 Oz Is Equal To How Many Cups
Mar 26, 2025
-
How Many Liters Is In 1500 Ml
Mar 26, 2025
-
Decay Of Carbon 14 By Beta Emission Equation
Mar 26, 2025
-
What Is The Square Root Of 192
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Sulfer Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
