Decay Of Carbon 14 By Beta Emission Equation
listenit
Mar 26, 2025 · 6 min read
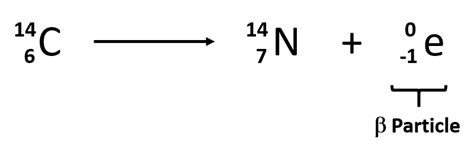
Table of Contents
The Decay of Carbon-14 by Beta Emission: A Comprehensive Guide
Carbon-14, a radioactive isotope of carbon, plays a crucial role in various scientific fields, most notably in radiocarbon dating. Its decay through beta emission is a fundamental process governed by precise nuclear physics principles. This article delves deep into the intricacies of carbon-14 decay, exploring the underlying equation, the half-life, applications, and limitations of this valuable radioactive isotope.
Understanding Beta Decay
Beta decay is a type of radioactive decay in which a beta particle (a high-energy electron or positron) is emitted from an atomic nucleus. This process alters the nucleus's composition, changing the number of protons and neutrons. Specifically, beta minus (β⁻) decay, relevant to carbon-14, involves the conversion of a neutron into a proton, emitting an electron and an antineutrino. This can be represented symbolically as:
n → p + e⁻ + ν̅ₑ
where:
- n represents a neutron
- p represents a proton
- e⁻ represents an electron (beta particle)
- ν̅ₑ represents an electron antineutrino
This transformation changes the atomic number of the element, increasing it by one, while the mass number remains the same. Therefore, carbon-14 (⁶C₁₄), with six protons and eight neutrons, decays into nitrogen-14 (⁷N₁₄), with seven protons and seven neutrons.
The Carbon-14 Decay Equation
The specific nuclear equation for the beta decay of carbon-14 is:
⁶C₁₄ → ⁷N₁₄ + e⁻ + ν̅ₑ
This equation concisely summarizes the transmutation: a carbon-14 nucleus transforms into a nitrogen-14 nucleus by emitting a beta particle (electron) and an electron antineutrino. The antineutrino, a nearly massless and chargeless particle, carries away some of the energy released during the decay process. The mass number (the sum of protons and neutrons) remains constant at 14, while the atomic number (number of protons) increases from 6 to 7. This change in atomic number is the key defining feature of beta decay.
The energy released during this decay is distributed between the beta particle and the antineutrino. The energy of the beta particle is not fixed; it varies continuously within a specific range, a characteristic feature of beta decay. This energy spectrum is determined by the energy levels involved in the nuclear transformation.
Kinetics of Carbon-14 Decay
The decay of carbon-14 follows first-order kinetics. This means the rate of decay is proportional to the number of carbon-14 atoms present at any given time. The decay can be described by the following equation:
N(t) = N₀e⁻λt
Where:
- N(t) is the number of carbon-14 atoms remaining after time t
- N₀ is the initial number of carbon-14 atoms
- λ is the decay constant, a characteristic of the isotope
- t is the time elapsed
The decay constant (λ) is related to the half-life (t₁/₂) of the isotope by the equation:
λ = ln(2) / t₁/₂
The half-life of carbon-14 is approximately 5,730 years. This means that after 5,730 years, half of the initial amount of carbon-14 will have decayed into nitrogen-14. After another 5,730 years, half of the remaining carbon-14 will have decayed, and so on.
Applications of Carbon-14 Decay: Radiocarbon Dating
The predictable and constant decay rate of carbon-14 makes it exceptionally useful for radiocarbon dating, a technique used to determine the age of organic materials up to around 50,000 years old. The method relies on the constant ratio of carbon-14 to carbon-12 in the atmosphere during the organism's lifetime. Living organisms continually exchange carbon with their environment, maintaining this equilibrium ratio. However, once an organism dies, this exchange stops, and the carbon-14 within it begins to decay.
By measuring the remaining ratio of carbon-14 to carbon-12 in a sample, scientists can estimate the time elapsed since the organism died. The lower the carbon-14 to carbon-12 ratio, the older the sample. Precise measurements of the isotope ratios are made using techniques like Accelerator Mass Spectrometry (AMS), which provides significantly higher sensitivity and allows dating of smaller samples.
Calibration Curves and Limitations of Radiocarbon Dating
While remarkably precise, radiocarbon dating requires calibration. The atmospheric concentration of carbon-14 has not been entirely constant throughout history. Variations due to solar activity, geomagnetic field fluctuations, and even human activities (like the burning of fossil fuels) influence the carbon-14 levels. Therefore, calibration curves, constructed using data from samples with known ages (e.g., tree rings, ice cores), are essential for accurate dating.
Several factors limit the accuracy and applicability of radiocarbon dating:
- Sample contamination: Contamination with younger or older carbon can significantly skew results.
- Reservoir effects: Organisms living in environments with different carbon-14 concentrations (e.g., marine organisms) may have differing initial ratios.
- Age limits: The method is generally limited to samples less than 50,000 years old, as the remaining carbon-14 becomes too small to measure accurately beyond this point.
- Sample size: Sufficient sample size is crucial for accurate measurement, especially with AMS techniques.
Beyond Radiocarbon Dating: Other Applications of Carbon-14
While radiocarbon dating is the most prominent application, carbon-14's radioactive properties also find use in other scientific areas:
- Tracing metabolic pathways: Radioactively labeled carbon-14 compounds can track metabolic processes in biological systems, providing insights into cellular functions.
- Environmental studies: Carbon-14 can monitor the movement and fate of carbon in the environment, contributing to our understanding of carbon cycles and climate change.
- Medical research: Though less common due to safety concerns, carbon-14 has been used in medical research to study drug metabolism and distribution within the body.
Safety Precautions when Handling Carbon-14
Carbon-14, like all radioactive isotopes, presents potential health hazards. While its beta emission is relatively low in energy and doesn't penetrate deeply into matter, prolonged exposure can still cause damage to cells and tissues. Therefore, appropriate safety measures must be followed when working with carbon-14, including:
- Minimizing exposure: Using remote handling equipment, minimizing handling time, and utilizing appropriate shielding are crucial.
- Appropriate personal protective equipment (PPE): Gloves, lab coats, and eye protection are essential.
- Proper disposal: Radioactive waste containing carbon-14 must be disposed of according to regulations.
The use of carbon-14 requires meticulous planning and adherence to established safety protocols to minimize risks to researchers and the environment.
Conclusion: The Enduring Importance of Carbon-14
The decay of carbon-14 through beta emission, as described by the equation ⁶C₁₄ → ⁷N₁₄ + e⁻ + ν̅ₑ, is a fundamental process with profound scientific implications. Its predictable decay rate forms the basis of radiocarbon dating, a powerful technique that has revolutionized our understanding of history and prehistory. While limitations exist, continued advancements in measurement techniques and calibration methods enhance the accuracy and expand the applications of this invaluable radioactive isotope. From archaeology to environmental science and medical research, carbon-14 continues to be a crucial tool in unraveling the mysteries of our world. Understanding its decay process and associated safety protocols ensures responsible and effective utilization of this powerful scientific asset. Further research continues to refine both the dating methodology and expand our understanding of the broader applications of this unique isotope within the scientific community. The ongoing exploration of its properties guarantees its continued importance in numerous scientific disciplines.
Latest Posts
Latest Posts
-
What Is Half Of A Half Teaspoon
Mar 29, 2025
-
What Percent Of 80 Is 15
Mar 29, 2025
-
Is Orange Juice A Heterogeneous Or Homogeneous Mixture
Mar 29, 2025
-
What Fraction Is Equivalent To 0 1 Repeating
Mar 29, 2025
-
Sound Waves Move The Slowest Through Which Medium
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Decay Of Carbon 14 By Beta Emission Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
