How Many Valence Electrons Does Ni Have
listenit
Mar 23, 2025 · 6 min read
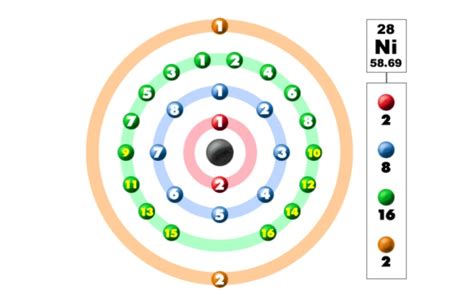
Table of Contents
How Many Valence Electrons Does Nickel (Ni) Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Nickel (Ni), a silvery-white metal with remarkable magnetic properties, plays a crucial role in various industries and natural processes. Understanding its atomic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and diverse applications. This comprehensive article delves into the electronic configuration of nickel, explains how to determine its valence electrons, and explores the implications of this characteristic on its reactivity and the compounds it forms.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we pinpoint the number of valence electrons in nickel, let's establish a foundational understanding of what valence electrons are and why they're so important. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of chemical bonds it can form. They dictate how an atom will interact with other atoms to achieve a stable electron configuration, typically resembling a noble gas.
The number of valence electrons is directly related to an element's position on the periodic table. This organization offers a powerful tool for predicting an element's properties, including its valence electron count. Understanding this relationship simplifies the process of determining the number of valence electrons for any element.
Determining Nickel's Electronic Configuration
To ascertain the number of valence electrons in nickel, we need to determine its electronic configuration. This configuration describes how electrons are distributed among different energy levels (shells) and sublevels (subshells) within an atom. Nickel's atomic number is 28, meaning it has 28 electrons.
The electronic configuration of nickel is written as 1s²2s²2p⁶3s²3p⁶4s²3d⁸. Let's break this down:
- 1s²: Two electrons in the first energy level (shell), specifically the s subshell.
- 2s²2p⁶: Eight electrons in the second energy level—two in the s subshell and six in the p subshell.
- 3s²3p⁶: Eight electrons in the third energy level—two in the s subshell and six in the p subshell.
- 4s²3d⁸: Ten electrons in the fourth energy level and the third energy level—two in the 4s subshell and eight in the 3d subshell.
Notice that the 4s subshell fills before the 3d subshell. This is a general trend, though there are exceptions. However, it’s important to remember that this filling order doesn't necessarily reflect the energy levels of the orbitals. In reality, the 3d and 4s subshells have very similar energies.
Identifying Nickel's Valence Electrons: The Outermost Shell
Now, let's identify nickel's valence electrons. Remember, valence electrons reside in the outermost shell. In nickel's case, the outermost shell is the fourth energy level (n=4). This shell contains two electrons in the 4s subshell. However, the situation is slightly more nuanced than just counting the 4s electrons.
While the 4s electrons are indeed the highest energy electrons, the 3d electrons also participate in bonding in many cases, especially in transition metals like nickel. This makes the determination of valence electrons for transition metals more complex than for main group elements.
Therefore, nickel is often considered to have two valence electrons (the 4s electrons), but also commonly exhibits variable valency. The 3d electrons, though not strictly in the outermost shell, are sufficiently close in energy to participate in chemical bonding, leading to nickel's ability to form compounds with various oxidation states (+2, +3, and even higher states in specific circumstances).
The Significance of Nickel's Variable Valency
The fact that nickel doesn't rigidly adhere to a fixed number of valence electrons is a defining characteristic of transition metals. This variable valency allows nickel to form a wide range of compounds with diverse properties.
The most common oxidation state for nickel is +2, resulting from the loss of the two 4s electrons. This leads to the formation of numerous nickel(II) compounds, such as nickel(II) oxide (NiO), nickel(II) chloride (NiCl₂), and nickel(II) sulfate (NiSO₄). These compounds demonstrate varied applications in different fields.
However, nickel can also exhibit a +3 oxidation state, and even higher states under specific conditions. This is due to the involvement of 3d electrons in bonding, resulting in compounds like nickel(III) oxide (Ni₂O₃), though these are generally less stable than nickel(II) compounds.
Nickel's Role in Chemistry and Industry
The variable valency of nickel is directly responsible for its diverse roles in both chemical reactions and industrial applications. Here are a few key areas:
-
Catalysis: Nickel's ability to participate in redox reactions, facilitated by its variable valency, makes it an excellent catalyst in various industrial processes. It's used in the hydrogenation of unsaturated fats (margarine production), in the synthesis of ammonia, and in various other catalytic conversions.
-
Alloying: Nickel is a crucial component in various alloys, enhancing their strength, corrosion resistance, and other desirable properties. It is found in stainless steel, nickel-chromium alloys (used in high-temperature applications), and other specialized alloys.
-
Batteries: Nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries utilize nickel's electrochemical properties to store and release electrical energy.
-
Magnetism: Nickel's unique electronic structure contributes to its ferromagnetic properties, making it useful in magnetic materials and applications.
-
Coatings: Nickel plating is widely used to protect metal surfaces from corrosion and improve their appearance.
Beyond Valence Electrons: Other Factors Influencing Nickel's Behavior
While the number of valence electrons is critical in understanding nickel's reactivity, other factors contribute to its overall chemical behavior. These include:
-
Atomic Radius: The size of the nickel atom influences its ability to form bonds and interact with other atoms.
-
Electronegativity: Nickel's electronegativity (its tendency to attract electrons in a chemical bond) determines the nature of the bonds it forms (ionic, covalent, metallic).
-
Ionization Energy: The energy required to remove an electron from a nickel atom influences its ability to lose electrons and form cations.
Conclusion: A Versatile Metal Defined by its Valence Electrons (and More)
Nickel's ability to form a variety of compounds with diverse oxidation states stems directly from its unique electronic configuration and its variable valency. While often considered to have two valence electrons (4s), the participation of 3d electrons in bonding broadens the spectrum of its chemical reactivity and explains its prominent role in diverse industrial processes and chemical reactions. Understanding the nuances of nickel's electronic structure provides a crucial insight into its extensive applications and crucial role in the modern world. This detailed explanation should clarify the number of valence electrons and enhance your overall understanding of this versatile metal.
Latest Posts
Latest Posts
-
How To Write Electron Configuration For Ions
Mar 25, 2025
-
How Many Feet Are In 0 4 Miles
Mar 25, 2025
-
What Do Letters Dna Stand For
Mar 25, 2025
-
What Is The Gcf Of 48 And 24
Mar 25, 2025
-
Molarity Of Acetic Acid In Vinegar
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Ni Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
