How Many Valence Electrons Does Ga Have
listenit
Mar 23, 2025 · 5 min read
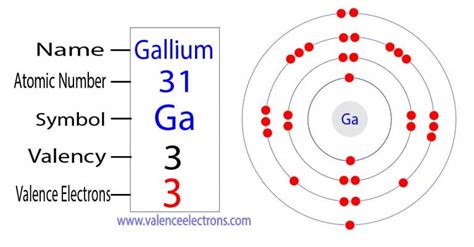
Table of Contents
How Many Valence Electrons Does Ga (Gallium) Have? Understanding Gallium's Electronic Structure and Reactivity
Gallium (Ga), a fascinating element residing in Group 13 of the periodic table, boasts a unique set of properties that make it crucial in various applications, from semiconductors to LEDs. A key aspect of understanding gallium's behavior lies in its electronic structure, specifically the number of valence electrons it possesses. This article delves deep into the electronic configuration of gallium, explaining why it has the number of valence electrons it does, and how this influences its chemical reactivity and applications.
Understanding Valence Electrons
Before focusing on gallium specifically, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they are the ones involved in chemical bonding. They determine an element's reactivity, the types of bonds it can form (ionic, covalent, metallic), and its overall chemical properties. The number of valence electrons an atom possesses usually dictates its position within the periodic table and its group number.
Gallium's Electronic Configuration: Unveiling the Mystery
Gallium's atomic number is 31, meaning it has 31 protons and, in a neutral atom, 31 electrons. To determine the number of valence electrons, we need to explore its electronic configuration. This configuration describes how electrons are distributed among different energy levels and subshells within the atom.
Gallium's electronic configuration is [Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>1</sup>. Let's break this down:
-
[Ar]: This represents the electron configuration of Argon (1s²2s²2p⁶3s²3p⁶), a noble gas. This core configuration signifies that gallium's inner shells are filled with electrons, similar to argon. These inner electrons are not involved in chemical bonding.
-
3d<sup>10</sup>: This indicates that the 3d subshell is completely filled with 10 electrons. These electrons are also considered core electrons and don't participate directly in chemical bonding.
-
4s<sup>2</sup>: This shows that the 4s subshell contains two electrons.
-
4p<sup>1</sup>: This reveals that the 4p subshell contains one electron.
Therefore, adding the electrons in the outermost shell (4s and 4p), we find that gallium has a total of 3 valence electrons. This is consistent with its position in Group 13 (or IIIA) of the periodic table, where elements typically have three valence electrons.
Why 3 Valence Electrons? The Significance of the Periodic Table
The periodic table is organized based on the electronic configurations of elements. Elements within the same group share similar valence electron configurations, resulting in similar chemical properties. Gallium's position in Group 13 reflects its three valence electrons. Elements in this group, such as boron (B), aluminum (Al), indium (In), and thallium (Tl), all exhibit a tendency to lose three electrons to achieve a stable octet configuration (eight electrons in their outermost shell), resembling the nearest noble gas.
Gallium's Reactivity: The Role of Valence Electrons
The three valence electrons determine gallium's reactivity. Gallium readily loses these three electrons to form Ga<sup>3+</sup> ions, exhibiting a +3 oxidation state. This tendency to lose electrons makes gallium a moderately reactive metal. However, it is less reactive than the alkali metals (Group 1) or alkaline earth metals (Group 2), which have only one or two valence electrons to lose, respectively.
The reactivity of gallium is influenced by factors beyond just the number of valence electrons. The size of the atom, the effective nuclear charge (the net positive charge experienced by valence electrons), and the shielding effect of inner electrons all contribute to its overall reactivity.
Applications of Gallium: From Semiconductors to Medicine
Gallium's unique electronic properties, stemming from its three valence electrons, underpin its diverse applications:
-
Semiconductors: Gallium arsenide (GaAs) is a crucial semiconductor material used in high-speed integrated circuits, LEDs, solar cells, and laser diodes. The ability of gallium to form covalent bonds with arsenic allows for the creation of materials with precisely controlled electrical conductivity.
-
LEDs (Light Emitting Diodes): Gallium nitride (GaN) and gallium-indium-nitride (GaInN) are vital components of high-efficiency LEDs, producing vibrant colors in various applications, from displays to lighting.
-
Medicine: Gallium-67 is a radioactive isotope used in medical imaging to detect infections and tumors. Its chemical behavior, dictated by its valence electrons, allows it to accumulate in areas of inflammation.
-
Alloys: Gallium is used in low-melting-point alloys, which find applications in fire detection systems and other temperature-sensitive technologies.
-
Other Applications: Gallium's unique properties extend to various other applications, including its use as a dopant in semiconductors, its role in the production of gallium mirrors, and its contribution to the development of new materials.
Comparing Gallium to Other Group 13 Elements
It's beneficial to compare gallium to other elements in Group 13 to further understand the influence of valence electrons on properties. While all these elements have three valence electrons, their properties vary slightly due to differences in atomic size and effective nuclear charge:
-
Boron (B): Boron is a metalloid with distinct properties different from the heavier Group 13 metals. Its smaller size and higher effective nuclear charge lead to different bonding behavior.
-
Aluminum (Al): Aluminum is a more reactive metal than gallium, due to its smaller size and less effective shielding of the valence electrons.
-
Indium (In) and Thallium (Tl): Indium and thallium are heavier than gallium and show some variations in their chemical reactivity and applications. The relativistic effects on heavier elements also influence their properties.
Conclusion: The Significance of Gallium's Three Valence Electrons
Gallium's three valence electrons are the cornerstone of its chemical behavior and a multitude of its applications. Its ability to readily lose these electrons to form Ga<sup>3+</sup> ions explains its moderate reactivity. The precise control of its electronic structure through chemical bonding with other elements enables the creation of crucial materials used in semiconductors, LEDs, and various other technologies. Understanding the electronic configuration and the role of valence electrons is essential for comprehending gallium's unique properties and its indispensable role in modern technology and medicine. Further research into gallium and its compounds continues to unlock new applications, highlighting the importance of this remarkable element with its defining three valence electrons.
Latest Posts
Latest Posts
-
How Many Seconds Are In 15 Years
Mar 25, 2025
-
10 To The Power Of Negative 5
Mar 25, 2025
-
How Many Seconds In 15 Years
Mar 25, 2025
-
How To Find The Mass Of Excess Reactant
Mar 25, 2025
-
Two Or More Atoms Combined Chemically
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Ga Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
