How To Find The Mass Of Excess Reactant
listenit
Mar 25, 2025 · 4 min read
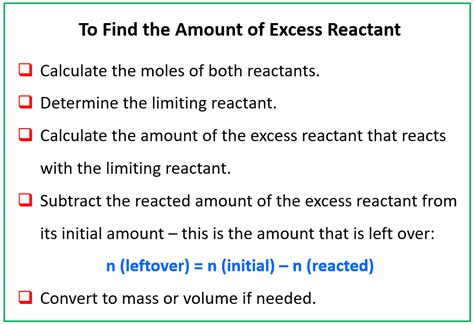
Table of Contents
How to Find the Mass of Excess Reactant: A Comprehensive Guide
Determining the mass of excess reactant is a crucial step in many stoichiometry problems. Understanding this concept is fundamental to mastering chemical calculations and interpreting experimental results. This comprehensive guide will walk you through the process, providing clear explanations, examples, and helpful tips to solidify your understanding.
Understanding Reactants and Limiting Reactants
Before diving into the calculation of excess reactants, let's revisit the basics. In a chemical reaction, reactants are the substances that undergo a chemical change to form products. Not all reactants are consumed completely during the reaction. The limiting reactant is the reactant that is completely consumed first, thus limiting the amount of product formed. Once the limiting reactant is used up, the reaction stops. Any remaining reactants are called excess reactants.
Identifying the Limiting Reactant
The first step in finding the mass of the excess reactant is to identify the limiting reactant. This can be done through several methods:
-
Mole Ratio Method: This method uses the balanced chemical equation to determine the mole ratio of reactants. You convert the mass of each reactant to moles using its molar mass, then compare the mole ratios to the stoichiometric ratios in the balanced equation. The reactant that produces fewer moles of product based on the stoichiometry is the limiting reactant.
-
Comparison of Product Yield: Calculate the theoretical yield of a product using each reactant individually. The reactant that yields the smallest amount of product is the limiting reactant.
Calculating the Mass of Excess Reactant: A Step-by-Step Approach
Let's illustrate the process with a detailed example:
Consider the reaction between 10.0 grams of hydrogen gas (H₂) and 50.0 grams of oxygen gas (O₂) to produce water (H₂O):
2H₂ + O₂ → 2H₂O
Step 1: Calculate the moles of each reactant:
-
Moles of H₂: The molar mass of H₂ is 2.02 g/mol. Therefore, moles of H₂ = (10.0 g) / (2.02 g/mol) = 4.95 moles
-
Moles of O₂: The molar mass of O₂ is 32.00 g/mol. Therefore, moles of O₂ = (50.0 g) / (32.00 g/mol) = 1.56 moles
Step 2: Determine the limiting reactant:
Using the balanced equation, we see that 2 moles of H₂ react with 1 mole of O₂. Let's find out how many moles of O₂ are needed to react completely with 4.95 moles of H₂:
(4.95 moles H₂) * (1 mole O₂ / 2 moles H₂) = 2.48 moles O₂
Since we only have 1.56 moles of O₂, O₂ is the limiting reactant. This means that all the O₂ will be consumed before all the H₂ is used up.
Step 3: Calculate the moles of excess reactant consumed:
Now, let's determine how many moles of H₂ react with the 1.56 moles of O₂:
(1.56 moles O₂) * (2 moles H₂ / 1 mole O₂) = 3.12 moles H₂
Step 4: Calculate the moles of excess reactant remaining:
We started with 4.95 moles of H₂ and 3.12 moles reacted. Therefore, the moles of H₂ remaining are:
4.95 moles - 3.12 moles = 1.83 moles
Step 5: Calculate the mass of excess reactant remaining:
Finally, we convert the moles of remaining H₂ to grams using its molar mass:
1.83 moles * 2.02 g/mol = 3.69 g
Therefore, the mass of the excess reactant (H₂) remaining is 3.69 grams.
Advanced Scenarios and Considerations
The process described above applies to most stoichiometry problems. However, let's explore some more complex scenarios:
Reactions with More Than Two Reactants
The same principles apply to reactions involving more than two reactants. You'll need to calculate the moles of each reactant and compare their ratios to the stoichiometric ratios in the balanced equation to identify the limiting reactant and subsequently calculate the mass of each excess reactant.
Dealing with Impurities
If your reactants contain impurities, you must account for the percentage purity when calculating the moles of the pure reactant. For example, if you have 10g of a reactant that is 90% pure, you would only use 9g (10g * 0.90) in your calculations.
Real-World Applications and Error Analysis
The calculation of excess reactants is not merely an academic exercise; it has numerous practical applications in various fields. From industrial chemical processes to pharmaceutical synthesis, accurately determining the amount of excess reactant is crucial for optimizing yields, controlling reaction conditions, and minimizing waste. Moreover, understanding potential sources of error in experimental measurements is essential for accurate interpretations.
Practical Tips and Troubleshooting
-
Always start with a balanced chemical equation: This is the foundation for all stoichiometric calculations. Ensure the equation is correctly balanced before proceeding.
-
Double-check your calculations: Carefully review each step to avoid arithmetic errors. Use a calculator and pay attention to significant figures.
-
Understand the context: Pay close attention to the units provided and ensure consistency throughout your calculations.
-
Practice regularly: The best way to master stoichiometry is through consistent practice. Work through various problems to build confidence and proficiency.
By following these steps and considering the advanced scenarios, you can accurately determine the mass of the excess reactant in any stoichiometry problem. Remember that a strong understanding of fundamental concepts, careful calculations, and attention to detail are key to success in this area of chemistry. Through persistent practice and application, you will master this important skill and confidently navigate complex chemical calculations.
Latest Posts
Latest Posts
-
Round Your Answer To The Nearest Cent
Mar 28, 2025
-
How Many Ounces Is 400 Mg
Mar 28, 2025
-
What Percent Is 40 Out Of 60
Mar 28, 2025
-
What 3 Subatomic Particles Make Up An Atom
Mar 28, 2025
-
What Is 3 6 As A Fraction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Mass Of Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
