Two Or More Atoms Combined Chemically
listenit
Mar 25, 2025 · 6 min read
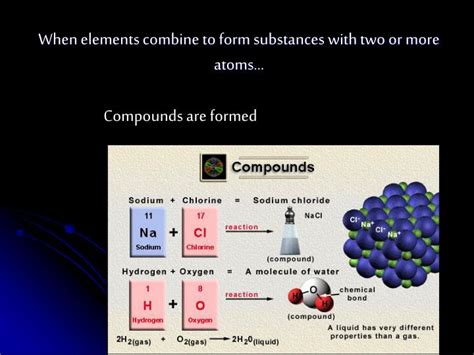
Table of Contents
When Atoms Unite: Exploring the World of Chemical Bonding
The world around us, from the air we breathe to the food we eat, is built from the fundamental building blocks of matter: atoms. However, rarely do we find atoms existing in isolation. Instead, they tend to combine with other atoms, forming a vast array of molecules and compounds through a process known as chemical bonding. Understanding chemical bonding is crucial to understanding the properties and behavior of all matter. This article will delve into the fascinating world of chemical bonding, exploring the different types of bonds, the forces involved, and the impact these bonds have on the macroscopic properties of substances.
The Driving Force Behind Bonding: Achieving Stability
Atoms bond with each other to achieve a more stable electron configuration. This typically involves achieving a full outer electron shell, a configuration that mimics the exceptionally stable electron arrangement of noble gases. This desire for stability is the driving force behind chemical bonding. Atoms will either gain, lose, or share electrons to reach this stable state. This quest for stability leads to a variety of bonding mechanisms.
The Major Types of Chemical Bonds:
Several types of chemical bonds exist, each characterized by distinct properties and interactions. The three primary types are:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds form when there's a significant difference in electronegativity between two atoms. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. In an ionic bond, one atom (typically a metal) loses one or more electrons, becoming a positively charged ion (cation), while another atom (typically a non-metal) gains these electrons, becoming a negatively charged ion (anion). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Characteristics of Ionic Compounds:
- High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome.
- Crystalline structure: Ions arrange themselves in a regular, repeating pattern forming a crystal lattice.
- Brittle: Slight shifts in the crystal lattice can cause like charges to align, leading to repulsion and fracture.
- Conduct electricity when molten or dissolved in water: Free-moving ions are capable of conducting an electric current.
Examples: Sodium chloride (NaCl), magnesium oxide (MgO), potassium iodide (KI).
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds form when two atoms share one or more pairs of electrons. This type of bonding occurs most frequently between non-metal atoms, where the electronegativity difference is small. The shared electrons are attracted to the nuclei of both atoms, holding them together.
Characteristics of Covalent Compounds:
- Lower melting and boiling points than ionic compounds: Covalent bonds are generally weaker than ionic bonds.
- Can be solids, liquids, or gases at room temperature: The strength of intermolecular forces (forces between molecules) influences the state of matter.
- Generally poor conductors of electricity: Electrons are localized within the covalent bond, not free to move.
- Can form large molecules: Covalent bonds can link many atoms together to create complex structures like proteins and DNA.
Types of Covalent Bonds:
- Nonpolar Covalent Bonds: Electrons are shared equally between atoms with similar electronegativities. Examples include diatomic molecules like H₂, O₂, and N₂.
- Polar Covalent Bonds: Electrons are shared unequally between atoms with different electronegativities. This creates a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals. In a metal, the valence electrons are delocalized; they're not associated with any particular atom but rather move freely throughout the metal lattice. This "sea" of electrons acts as a glue, holding the positively charged metal ions together.
Characteristics of Metallic Compounds:
- High electrical and thermal conductivity: The free-moving electrons readily carry both electricity and heat.
- Malleable and ductile: The sea of electrons allows metal ions to slide past each other without disrupting the bonding.
- Lustrous: The delocalized electrons can absorb and re-emit light, giving metals their characteristic shine.
- Variable melting and boiling points: Depending on the strength of the metallic bond.
Examples: Iron (Fe), copper (Cu), gold (Au).
Beyond the Basics: Exploring Other Bonding Interactions
While ionic, covalent, and metallic bonds are the primary types, several other weaker interactions significantly impact the properties of substances.
Hydrogen Bonding: A Special Case of Dipole-Dipole Interaction
Hydrogen bonds are a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule. This interaction is responsible for many unique properties of water, including its high boiling point and surface tension.
Van der Waals Forces: Weak but Ubiquitous
Van der Waals forces are weak, short-range attractive forces between molecules. They arise from temporary fluctuations in electron distribution around atoms and molecules. While individually weak, they can collectively contribute significantly to the properties of substances, especially those with low molecular weights. These forces include:
- London Dispersion Forces: Present in all molecules, these forces result from temporary dipoles induced by electron fluctuations.
- Dipole-Dipole Forces: Occur between polar molecules, where the positive end of one molecule attracts the negative end of another.
The Impact of Chemical Bonds on Macroscopic Properties:
The type of chemical bond significantly influences the macroscopic properties of a substance. For example, the strong ionic bonds in sodium chloride lead to its high melting point and crystalline structure, while the weaker covalent bonds in water allow it to exist as a liquid at room temperature. The delocalized electrons in metals account for their excellent electrical conductivity. Understanding the relationship between bonding and properties is essential in materials science and chemical engineering.
Conclusion: A Foundation of Chemistry
Chemical bonding is a fundamental concept in chemistry, providing the framework for understanding the structure and behavior of matter. From the simple formation of diatomic molecules to the complexity of biological macromolecules, the principles of chemical bonding remain central to our understanding of the natural world. Further exploration of this topic can lead to a deeper appreciation of the intricate dance of atoms and the remarkable diversity of materials that result from their interactions. Further research into specific types of bonding, the influence of bond strength on reactivity, and the applications of bonding principles in different fields will only enhance your understanding of this crucial chemical concept. The world of chemical bonding is a vast and fascinating one, full of intricate details and far-reaching implications, waiting to be further explored.
Latest Posts
Latest Posts
-
What 3 Subatomic Particles Make Up An Atom
Mar 28, 2025
-
What Is 3 6 As A Fraction
Mar 28, 2025
-
How Many Cups In 1 2 Gal
Mar 28, 2025
-
39 Is What Percent Of 12 5
Mar 28, 2025
-
During Which Stage Of Cell Cycle Does Dna Replication Occur
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Two Or More Atoms Combined Chemically . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
