How Many Valence Electrons Do The Alkaline Metals Have
listenit
Mar 27, 2025 · 5 min read
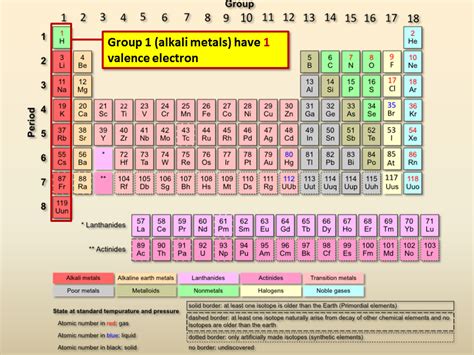
Table of Contents
How Many Valence Electrons Do the Alkaline Metals Have? A Deep Dive into Group 1 Elements
The alkaline metals, a vibrant and reactive group in the periodic table, hold a special place in chemistry. Understanding their properties, especially their valence electron configuration, is key to comprehending their behavior and the role they play in various chemical reactions. This comprehensive article delves deep into the question: How many valence electrons do alkaline metals have? We'll explore the concept of valence electrons, examine the electronic structure of alkaline metals, and uncover how this single valence electron dictates their characteristic properties and reactivity.
Understanding Valence Electrons: The Key to Reactivity
Before we pinpoint the number of valence electrons in alkaline metals, let's establish a firm grasp on what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the furthest from the atom's nucleus and are therefore the most loosely bound. This loose binding is what makes them crucial for chemical bonding. They participate directly in chemical reactions, determining an element's reactivity and the types of bonds it can form.
The number of valence electrons an atom possesses directly influences its chemical behavior. Atoms strive for stability, often achieved by having a full outer electron shell (octet rule, except for hydrogen and helium). Atoms with nearly full or nearly empty outer shells are more likely to react with other atoms to gain or lose electrons and achieve this stable configuration.
The Alkaline Metals: A Family Portrait
The alkaline metals, belonging to Group 1 of the periodic table (excluding hydrogen), comprise lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These elements share striking similarities in their physical and chemical properties, primarily due to their identical valence electron configuration. Their electronic structure is the foundation of their characteristic reactivity.
The Defining Characteristic: One Valence Electron
The answer to our central question is straightforward: alkaline metals possess only one valence electron. This single electron residing in their outermost s-orbital is the cornerstone of their chemical behavior and shared properties. This lone electron is easily lost, leading to the formation of a +1 ion (cation) and explaining their high reactivity.
Let's illustrate this with the electronic configurations of some alkaline metals:
- Lithium (Li): 1s² 2s¹ (One valence electron in the 2s orbital)
- Sodium (Na): 1s² 2s² 2p⁶ 3s¹ (One valence electron in the 3s orbital)
- Potassium (K): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ (One valence electron in the 4s orbital)
Notice the pattern: regardless of the number of inner electron shells, each alkaline metal has only one electron in its outermost shell – its valence electron. This consistent electronic structure is the reason for their similar chemical properties.
The Impact of One Valence Electron: Explaining Alkaline Metal Properties
The presence of only one valence electron profoundly impacts the properties of alkaline metals. Here's how:
1. High Reactivity:
The single valence electron is easily lost, forming a stable +1 ion. This explains their high reactivity, particularly with nonmetals like halogens (Group 17). Alkaline metals readily react with water, producing hydrogen gas and a metal hydroxide, often with vigorous reactions. For example, sodium reacts violently with water, producing a significant amount of heat.
2. Low Ionization Energies:
Removing the single valence electron requires relatively little energy, resulting in low ionization energies. This ease of electron removal contributes to their high reactivity and the formation of stable cations.
3. Low Electronegativity:
Alkaline metals exhibit low electronegativity, meaning they have a weak attraction for electrons. They readily lose their valence electron rather than gaining one, further emphasizing their tendency to form cations.
4. Metallic Bonding and Properties:
The valence electrons are delocalized, forming a "sea" of electrons that hold the metal cations together through metallic bonding. This bonding explains the characteristic metallic properties of alkaline metals: good electrical conductivity, good thermal conductivity, malleability, and ductility.
5. Reactivity Trends within the Group:
While all alkaline metals share the same valence electron configuration, their reactivity increases as you move down the group. This is because the outermost electron is further from the nucleus and therefore less strongly attracted, making it easier to lose. Hence, cesium (Cs) is the most reactive alkaline metal.
Applications of Alkaline Metals: Leveraging Their Reactivity
The unique properties of alkaline metals, stemming from their single valence electron, make them valuable in various applications:
-
Lithium-ion batteries: Lithium's high electrochemical potential makes it ideal for use in high-energy-density batteries found in electronic devices and electric vehicles.
-
Sodium lamps: Sodium vapor lamps emit intense yellow light, making them efficient for street lighting.
-
Potassium in fertilizers: Potassium is an essential nutrient for plant growth, and potassium salts are crucial components of many fertilizers.
-
Rubidium and Cesium in Atomic Clocks: Their precise atomic transitions allow for the creation of highly accurate atomic clocks.
Conclusion: The Single Valence Electron's Significance
The answer to "How many valence electrons do the alkaline metals have?" is unequivocally one. This seemingly simple fact underpins the remarkable reactivity and unique properties of these elements. Their single valence electron dictates their chemical behavior, influencing their reactions, their ability to form stable cations, and their applications in various technologies. Understanding the significance of this single electron is essential for grasping the fundamental principles of chemical bonding and the properties of matter. Further exploration into the specific reactions and applications of each alkaline metal reveals a fascinating array of chemical phenomena dictated by this fundamental characteristic.
Latest Posts
Latest Posts
-
What Is 39 6 Celsius In Fahrenheit
Mar 30, 2025
-
44 Is 55 Of What Number
Mar 30, 2025
-
How Does Price Discrimination Benefit Producers And Consumers
Mar 30, 2025
-
Which Element Is The Most Metallic
Mar 30, 2025
-
Do Srtrong Bases Completely Dissociate In Water
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do The Alkaline Metals Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
