Which Element Is The Most Metallic
listenit
Mar 30, 2025 · 5 min read
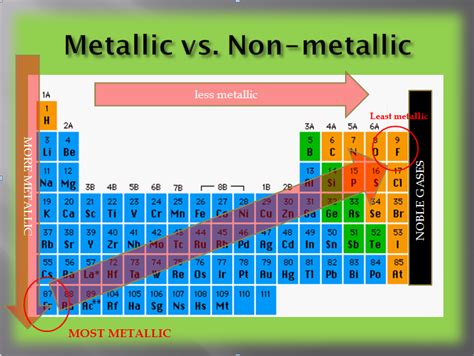
Table of Contents
Which Element is the Most Metallic? A Deep Dive into Electronegativity, Ionization Energy, and Metallic Character
The question of which element is the "most metallic" isn't a simple one with a single definitive answer. Metallic character isn't a single, directly measurable property like mass or density. Instead, it's a collection of properties that work together to define how "metallic" an element behaves. To understand which element holds the title, we need to delve into the key properties that contribute to metallic character: electronegativity, ionization energy, and atomic radius.
Understanding Metallic Character: A Blend of Properties
Metallic character describes the tendency of an element to exhibit properties characteristic of metals. These properties include:
- Electrical Conductivity: Metals readily conduct electricity due to the free movement of electrons in their delocalized electron sea.
- Thermal Conductivity: Metals efficiently transfer heat, again due to the mobile electrons.
- Malleability and Ductility: Metals can be hammered into sheets (malleability) and drawn into wires (ductility) without breaking, a result of the non-directional nature of metallic bonding.
- Luster: Metals typically have a shiny appearance, reflecting light.
- Low Ionization Energy: Metals readily lose electrons to form positive ions (cations).
- Low Electronegativity: Metals have a weak attraction for electrons.
These properties are interconnected and arise from the unique electronic structure of metals. The outer valence electrons are loosely held and readily participate in metallic bonding, a sea of delocalized electrons shared among a lattice of positively charged metal ions.
Key Indicators of Metallic Character
Several properties are crucial in determining the degree of metallic character:
1. Electronegativity: The Tug-of-War for Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Elements with low electronegativity are more metallic. They don't strongly attract electrons and readily lose them to form positive ions. The trend in the periodic table shows electronegativity increasing across a period (left to right) and decreasing down a group (top to bottom). Thus, elements in the bottom left corner of the periodic table generally have the lowest electronegativity.
2. Ionization Energy: The Energy Cost of Losing Electrons
Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Metals have low ionization energies, meaning it's relatively easy to remove electrons from them. The lower the ionization energy, the more metallic the element. Similar to electronegativity, ionization energy decreases down a group and increases across a period.
3. Atomic Radius: The Size Matters
Atomic radius is a measure of the size of an atom. Larger atoms generally exhibit greater metallic character. This is because the outermost electrons are further from the nucleus and experience a weaker electrostatic attraction, making them easier to lose and contribute to metallic bonding. Atomic radius increases down a group and decreases across a period.
The Contenders for the "Most Metallic" Title: Alkali Metals and Alkaline Earth Metals
Based on the trends in electronegativity, ionization energy, and atomic radius, the elements most likely to be considered the "most metallic" are found within the alkali metals (Group 1) and alkaline earth metals (Group 2) of the periodic table.
Alkali metals (Li, Na, K, Rb, Cs, Fr) exhibit exceptionally low electronegativity and ionization energy and have relatively large atomic radii. They are highly reactive, readily losing one electron to form +1 ions. Francium (Fr), the heaviest alkali metal, is often cited as the most metallic element due to its exceptionally low electronegativity and ionization energy and its large atomic radius. However, its extreme radioactivity and rarity make its properties difficult to study extensively.
Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) are also highly metallic, although less so than the alkali metals. They readily lose two electrons to form +2 ions, possessing lower electronegativity and ionization energy than most other elements but higher than the alkali metals. Radium (Ra), the heaviest alkaline earth metal, exhibits higher metallic character than other members of its group. However, it is also highly radioactive and rare, hindering comprehensive study.
Beyond Electronegativity, Ionization Energy, and Atomic Radius: Other Factors
While electronegativity, ionization energy, and atomic radius are primary indicators, other factors subtly influence metallic character:
- Crystal Structure: The arrangement of atoms in a metal's crystal lattice can affect its properties.
- Density: Denser metals often have stronger metallic bonding.
- Melting and Boiling Points: These points reflect the strength of metallic bonds.
- Electrical and Thermal Conductivity: These properties directly reflect the mobility of electrons in the metallic structure.
These factors add complexity to the simple ranking based on electronegativity, ionization energy, and atomic radius.
The Verdict: No Single "Most Metallic" Element
There's no universally agreed-upon "most metallic" element. While francium (Fr) and radium (Ra) often top the list based on their low electronegativity, ionization energy, and large atomic radii, their extreme radioactivity and scarcity make them challenging to study comprehensively. Therefore, the title of "most metallic" is more of a relative term based on the collective properties of an element rather than a definitive, measurable quantity.
Conclusion: A Spectrum of Metallic Character
It is more accurate to think of metallic character as existing on a spectrum rather than assigning a single element as the "most metallic." The alkali metals and alkaline earth metals reside at the highly metallic end of this spectrum, with francium and radium frequently cited as leading contenders based on available data. However, the extreme radioactivity and scarcity of these elements warrant a nuanced understanding of their metallic properties and careful consideration of the broader factors that influence metallic character. Further research and advancements in studying highly radioactive elements could refine our understanding and potentially shift our perspective on which element best embodies the characteristics of a "most metallic" element.
Latest Posts
Latest Posts
-
What Are Convection Currents And What Causes Them
Apr 01, 2025
-
Circumference Of A Circle With A Diameter Of 10
Apr 01, 2025
-
What Transition Metals Have A Fixed Charge
Apr 01, 2025
-
What Are Biotic Factors And Abiotic Factors
Apr 01, 2025
-
Common Multiples Of 4 And 9
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Element Is The Most Metallic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
