How Many Valence Electrons Are In I
listenit
Apr 01, 2025 · 5 min read
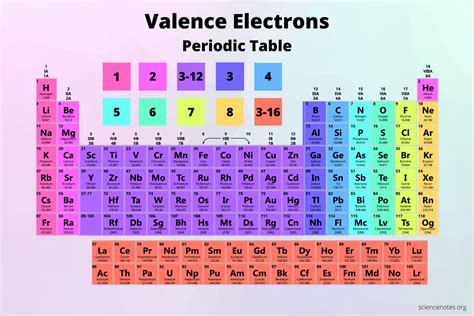
Table of Contents
How Many Valence Electrons are in Iodine? Understanding Iodine's Chemical Behavior
Iodine, a fascinating element with a rich history and diverse applications, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to grasping its chemical behavior and reactivity. This comprehensive article delves deep into the world of iodine, explaining its valence electron count, its implications for bonding, and its various applications.
What are Valence Electrons?
Before we dive into iodine's valence electrons, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's chemical properties and how it interacts with other elements.
Determining Iodine's Valence Electrons
Iodine (I) is a halogen located in Group 17 (VIIA) of the periodic table. The group number itself provides a significant clue to the number of valence electrons. Group 17 elements, also known as halogens, characteristically possess seven valence electrons. This is because their electron configuration generally ends in ns²np⁵, where n represents the principal quantum number. This configuration leaves them one electron short of a stable, filled outer shell, making them highly reactive and prone to forming one covalent bond to achieve a stable octet.
Iodine's Electronic Configuration
To solidify our understanding, let's examine iodine's electronic configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵. Notice that the outermost shell (the 5th shell) contains 2 electrons in the 5s subshell and 5 electrons in the 5p subshell. Adding these together (2 + 5 = 7), we confirm that iodine indeed has seven valence electrons.
Implications of Iodine's Seven Valence Electrons
The presence of seven valence electrons significantly impacts iodine's chemical behavior. This near-complete outer shell drives its strong tendency to gain one electron to achieve a stable octet configuration, resembling the noble gas xenon. This tendency leads to several key characteristics:
1. Formation of Ionic Bonds
Iodine readily forms ionic bonds with electropositive elements, such as alkali metals and alkaline earth metals. In these bonds, iodine gains an electron, acquiring a -1 charge and forming an iodide ion (I⁻). The electrostatic attraction between the positively charged metal ion and the negatively charged iodide ion constitutes the ionic bond. For example, the reaction between sodium (Na) and iodine yields sodium iodide (NaI):
2Na(s) + I₂(s) → 2NaI(s)
2. Formation of Covalent Bonds
Iodine can also form covalent bonds with other nonmetals, sharing electrons to achieve a stable octet. This type of bonding is prevalent in iodine's numerous compounds. For instance, in iodine monochloride (ICl), iodine shares one electron with chlorine to complete its octet. The strength of covalent bonds involving iodine can vary depending on the electronegativity of the other atom.
3. Oxidizing and Reducing Properties
Due to its ability to easily gain an electron, iodine acts as an oxidizing agent. It readily accepts electrons from other species, causing them to be oxidized. However, iodine can also exhibit reducing properties under certain conditions, especially when reacting with stronger oxidizing agents. This dual nature contributes to its role in various redox reactions.
Iodine's Applications: A Valence Electron Perspective
The unique chemical properties stemming from its seven valence electrons enable iodine to find widespread applications in various fields.
1. Medical Applications: Iodine in Healthcare
Iodine's crucial role in thyroid hormone production underscores its significance in human health. The thyroid gland utilizes iodine to synthesize thyroxine (T4) and triiodothyronine (T3), hormones responsible for regulating metabolism, growth, and development. Iodine deficiency can lead to serious health problems like goiter and hypothyroidism. Iodine-containing compounds are used in medical treatments, including disinfectants and antiseptics. The antiseptic properties of iodine are a direct consequence of its high reactivity, enabling it to disrupt the cellular processes of microorganisms.
2. Industrial Applications: Catalysis and other uses
Iodine compounds find significant use in industrial settings, often as catalysts. Their ability to participate in redox reactions makes them suitable for specific chemical processes. For instance, iodine is utilized in certain organic syntheses and in the production of various chemicals. Iodine's interaction with other substances, guided by its valence electrons' behavior, underpins its catalytic action.
3. Analytical Chemistry: Iodine's Role in Titrations
Iodine's reactivity makes it a valuable tool in analytical chemistry. It's involved in redox titrations, where its precise reaction with other substances allows for accurate quantitative analysis. The predictable behavior of iodine, derived from its electron configuration and valence electrons, ensures the reliability of these titration methods.
Beyond the Octet Rule: Exceptions and Complexities
While the octet rule provides a useful framework for understanding bonding, there are exceptions, and iodine exhibits some of these. In certain compounds, iodine can exceed an octet, meaning it can have more than eight electrons in its valence shell. This is especially true for iodine compounds involving higher oxidation states. This expansion of the valence shell is a consequence of the availability of empty d orbitals in iodine's valence shell, allowing for the accommodation of extra electrons.
Conclusion: The Significance of Valence Electrons in Iodine Chemistry
In conclusion, iodine's seven valence electrons are the key to understanding its multifaceted chemical behavior. This feature drives its reactivity, determining its ability to form ionic and covalent bonds and its roles as both an oxidizing and reducing agent. These properties have profound implications for iodine's diverse applications in medicine, industry, and analytical chemistry. The study of iodine's valence electrons serves as a fundamental example of how an element's electronic structure dictates its chemical properties and its significance in the world around us. Further research continually unveils new facets of iodine's chemistry, reinforcing the importance of understanding its fundamental electronic configuration. From its role in human physiology to its industrial applications, iodine's significance is intimately tied to the properties shaped by its seven valence electrons. Understanding this fundamental aspect of iodine's nature allows us to appreciate its crucial role in various fields and further explore its potential applications.
Latest Posts
Latest Posts
-
What Is The Bottom Of A Wave Called
Apr 02, 2025
-
Greatest Common Factor Of 18 And 30
Apr 02, 2025
-
Miles Per Hour To Meters Per Minute
Apr 02, 2025
-
Has A Definite Volume And Shape
Apr 02, 2025
-
What Is 3 Out Of 25 As A Percentage
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In I . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
